Crystal Structure of O-Acetylserine Sulfhydrylase from Haemophilus inuen-zae in complex with pre-reactive o-acetyl serine and peptide inhibitor
Ekka, M.K., Singh, A.K., Kaushik, A., Kumaran, S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Cysteine synthase | A [auth X] | 332 | Haemophilus influenzae Rd KW20 | Mutation(s): 0 Gene Names: cysK, HI_1103 EC: 2.5.1.47 | 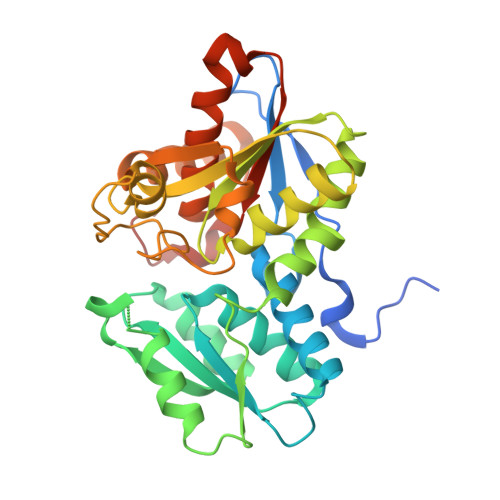 |
UniProt | |||||
Find proteins for P45040 (Haemophilus influenzae (strain ATCC 51907 / DSM 11121 / KW20 / Rd)) Explore P45040 Go to UniProtKB: P45040 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P45040 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| C-terminal peptide from Serine acetyltransferase | 10 | Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 | Mutation(s): 0 EC: 2.3.1.30 | 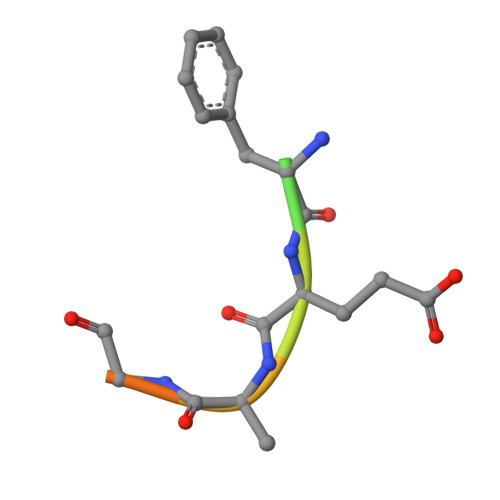 | |
UniProt | |||||
Find proteins for P29847 (Salmonella typhimurium (strain LT2 / SGSC1412 / ATCC 700720)) Explore P29847 Go to UniProtKB: P29847 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P29847 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PLP Query on PLP | D [auth X] | PYRIDOXAL-5'-PHOSPHATE C8 H10 N O6 P NGVDGCNFYWLIFO-UHFFFAOYSA-N |  | ||
| OAS Query on OAS | C [auth X] | O-ACETYLSERINE C5 H9 N O4 VZXPDPZARILFQX-BYPYZUCNSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 112.634 | α = 90 |
| b = 112.634 | β = 90 |
| c = 43.49 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHASER | phasing |