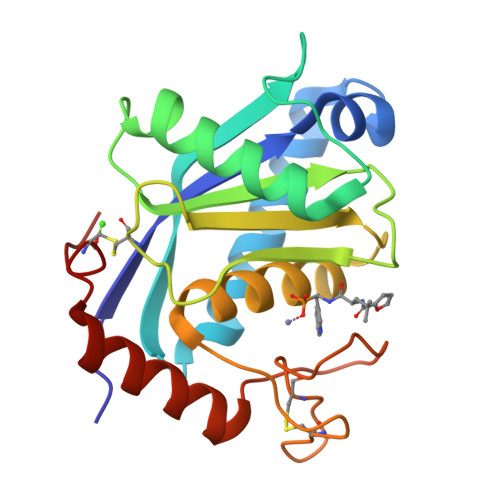
2 angstrom X-ray structure of adamalysin II complexed with a peptide phosphonate inhibitor adopting a retro-binding mode.
Cirilli, M., Gallina, C., Gavuzzo, E., Giordano, C., Gomis-Ruth, F.X., Gorini, B., Kress, L.F., Mazza, F., Paradisi, M.P., Pochetti, G., Politi, V.(1997) FEBS Lett 418: 319-322
- PubMed: 9428736
- DOI: https://doi.org/10.1016/s0014-5793(97)01401-4
- Primary Citation of Related Structures:
4AIG - PubMed Abstract:
The search of reprolysin inhibitors offers the possibility of intervention against both matrixins and ADAMs. Here we report the crystal structure of the complex between adamalysin II, a member of the reprolysin family, and a phosphonate inhibitor modeled on an endogenous venom tripeptide. The inhibitor occupies the primed region of the cleavage site adopting a retro-binding mode. The phosphonate group ligates the zinc ion in an asymmetric bidentate mode and the adjacent Trp indole system partly fills the primary specificity subsite S1'. An adamalysin-based model of tumor necrosis factor-alpha-converting enzyme (TACE) reveals a smaller S1' pocket for this enzyme.
Organizational Affiliation:
Ist. Strutturistica Chimica, CNR, Rome, Italy.