Structural basis for the subtype-selective activation of KCa3.1 channels.
Nam, Y.W., Ramanishka, A., Zhang, M.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Intermediate conductance calcium-activated potassium channel protein 4 | 340 | Homo sapiens | Mutation(s): 0 Gene Names: KCNN4, IK1, IKCA1, KCA4, SK4 | 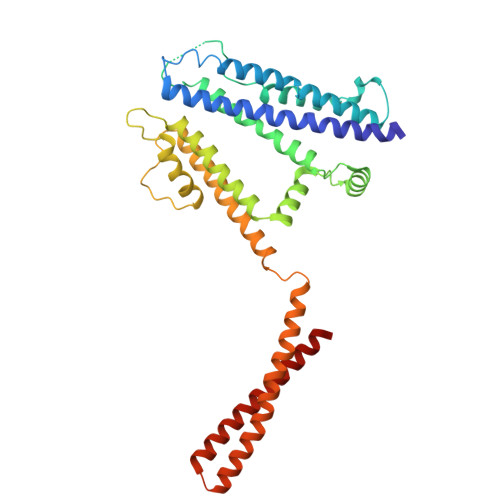 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15554 (Homo sapiens) Explore O15554 Go to UniProtKB: O15554 | |||||
PHAROS: O15554 GTEx: ENSG00000104783 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15554 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Calmodulin-1 | 67 | Homo sapiens | Mutation(s): 0 Gene Names: CALM1, CALM, CAM, CAM1 | 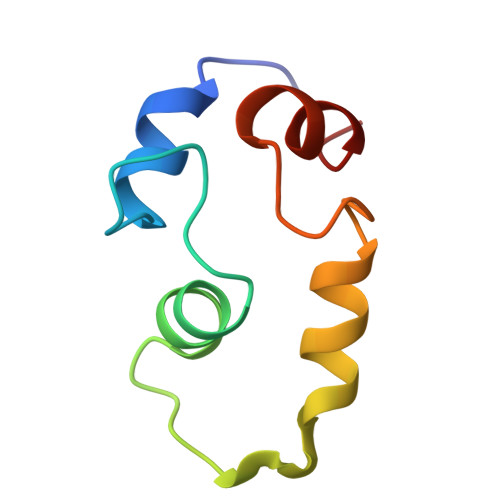 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0DP23 (Homo sapiens) Explore P0DP23 Go to UniProtKB: P0DP23 | |||||
PHAROS: P0DP23 GTEx: ENSG00000198668 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0DP23 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| K Query on K | I [auth A], J [auth A], K [auth A], L [auth A] | POTASSIUM ION K NPYPAHLBTDXSSS-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.21.1_5286 |
| RECONSTRUCTION | cryoSPARC |
| Funding Organization | Location | Grant Number |
|---|---|---|
| American Heart Association | United States | 23AIREA1039423 |
| American Heart Association | United States | 24CDA1260237 |
| National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) | United States | 4R33 NS101182-03 |
| National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) | United States | R15 NS130420-01A1 |