Post Acquisition Super Resolution for Cryo-Electron Microscopy
Burton-Smith, R.N., Murata, K.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ferritin heavy chain, N-terminally processed | 174 | Mus musculus | Mutation(s): 0 Gene Names: Fth1, Fth EC: 1.16.3.1 | 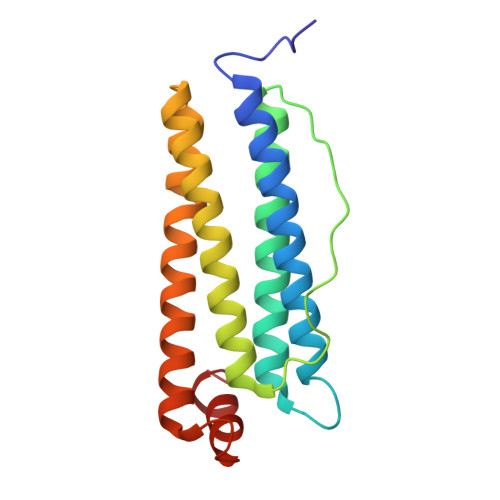 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P09528 (Mus musculus) Explore P09528 Go to UniProtKB: P09528 | |||||
IMPC: MGI:95588 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P09528 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FE Query on FE | AA [auth C] BA [auth E] CA [auth L] DA [auth P] Y [auth A] | FE (III) ION Fe VTLYFUHAOXGGBS-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| RECONSTRUCTION | RELION |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Japan Society for the Promotion of Science (JSPS) | Japan | -- |