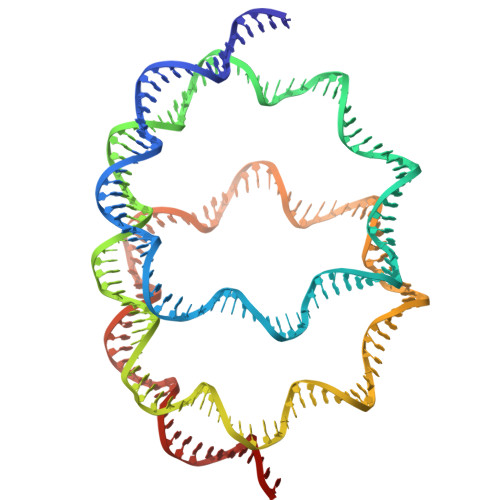
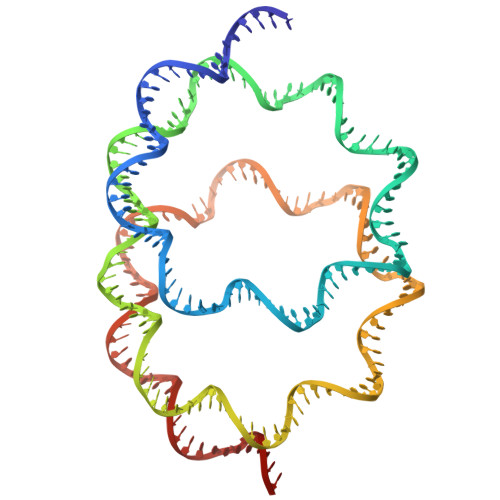
A method for cryo-EM analysis of eukaryotic nucleosomes reconstituted in bacterial cells.
Ho, C.H., Kobayashi, Y., Ogasawara, M., Takizawa, Y., Kurumizaka, H.(2026) iScience 29: 114453-114453
- PubMed: 41541688
- DOI: https://doi.org/10.1016/j.isci.2025.114453
- Primary Citation of Related Structures:
9W74 - PubMed Abstract:
Conventional methods for preparing nucleosomes are time-consuming and technically demanding. In the present study, we extended the approach of generating nucleosomes in Escherichia coli by the co-expression of all four histones, allowing nucleosomes to be assembled in cells. The bacterially reconstituted nucleosomes can be readily prepared from the E. coli cells and directly subjected to cryo-EM single particle analysis. Using this method, we obtained a 2.56 Å nucleosome structure that is highly similar to a previously reported nucleosome crystal structure, validating the use of nucleosomes formed in E. coli for cryo-EM analysis. Unexpectedly, we also discovered a non-canonical nucleosome structure, in which two hexasomes are closely packed. This system provides a robust and efficient platform for structural studies of nucleosomes and nucleosome variants, and may facilitate the discovery of chromatin architectures.
- Laboratory of Chromatin Structure and Function, Institute for Quantitative Biosciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-0032, Japan.
Organizational Affiliation: