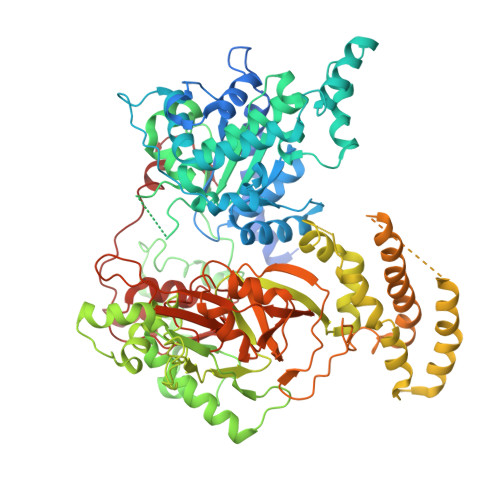
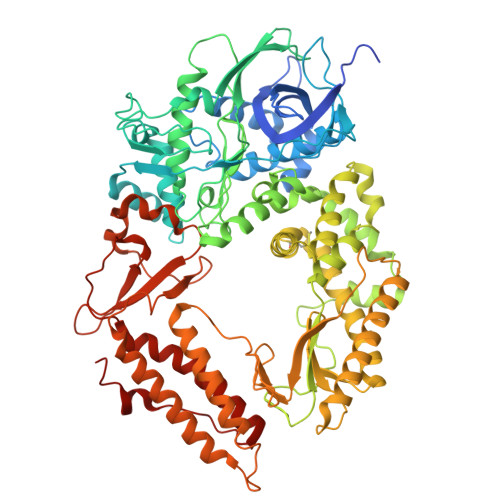
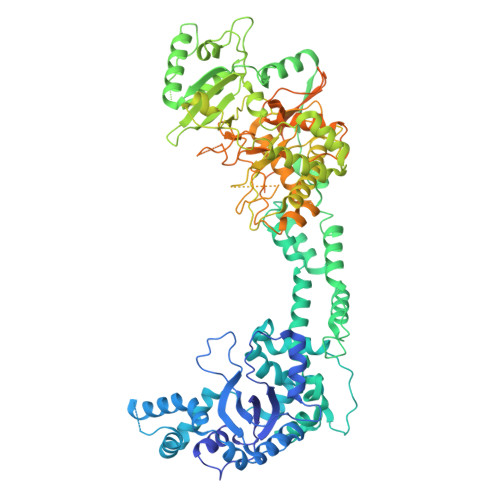
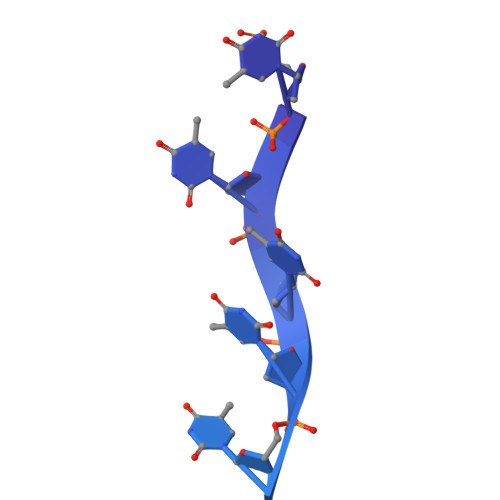
Structural and mechanistic insights into the herpes simplex virus type 1 helicase-primase primosome.
Wu, Y., Jiang, Z., Chen, X., Li, D., Zhang, Z., Dong, C.(2025) Cell Discov 11: 100-100
- PubMed: 41372195
- DOI: https://doi.org/10.1038/s41421-025-00855-4
- Primary Citation of Related Structures:
9VLQ - PubMed Abstract:
DNA unwinding and primer synthesis are fundamental processes in genome replication. The human herpes simplex virus type 1 (HSV-1) helicase-primase forms a unique heterotrimeric primosome that is essential for viral DNA unwinding and primer synthesis and represents an ideal drug target. However, its molecular mechanism remains poorly understood. Here we report the cryo-electron microscopic structure of the primosome in complex with single-stranded DNA, ADP and Mg 2+ to 3.47 Å resolution, which reveals that the primosome forms an unprecedented architecture in a fully open DNA binding groove between the helicase domains 1A and 2A-2B and that the primase subunit UL52 interacts extensively with the helicase subunit UL5 and accessory protein subunit UL8. Integrating mutagenesis, biochemical assays, structural analysis and 3D variability display analysis, we have identified the active sites of the ATPase, helicase and primase and critical interfaces between UL52, UL5 and UL8. Our work suggests that the primosome unwinds and translocates DNA via bidirectional rotation, and proposes a mechanistic model for DNA-dependent ATPase activation and alternating activity between helicase and primase. Herpesviridae family viruses pose significant threats to human health worldwide, and this trimeric assembly of primosomes is conserved. Our work provides a framework for understanding replication mechanisms across related viruses and for the rational design of broad-spectrum antivirals.
- Department of Thyroid and Breast Surgery, Zhongnan Hospital of Wuhan University, School of Pharmaceutical Sciences, Wuhan University, Wuhan, Hubei, China.
Organizational Affiliation: