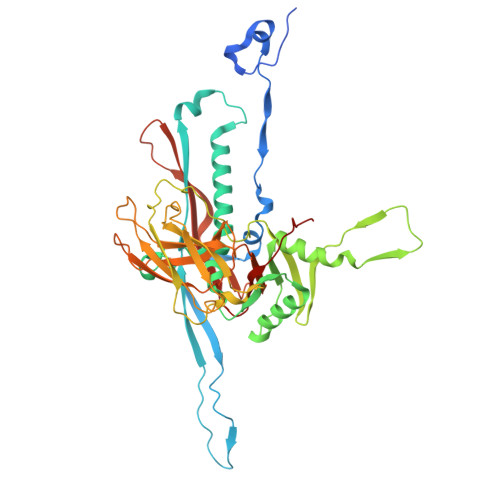
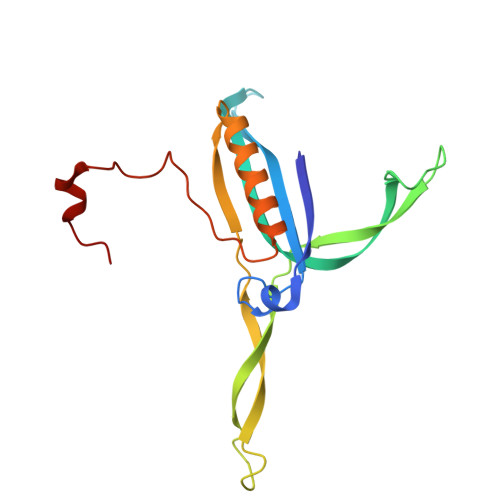
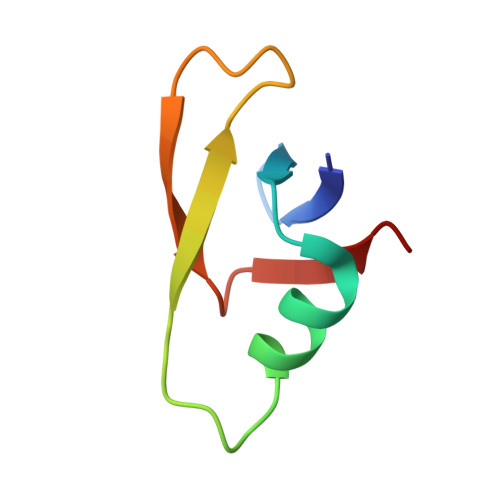
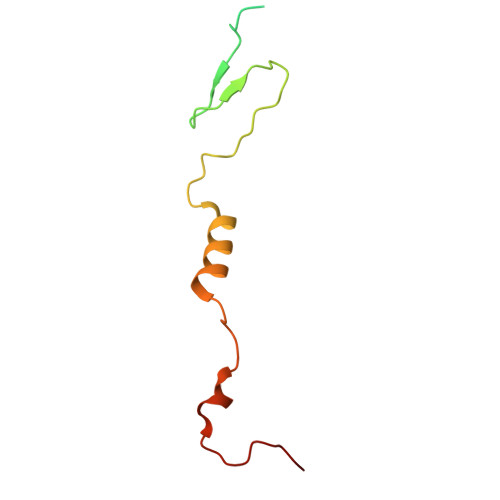
Capsid structure of phage SPO1 reveals novel minor capsid proteins and insights into capsid stabilization.
Zhao, X., Wang, A., Wang, Y., Kang, Y., Shao, Q., Li, L., Zheng, Y., Hu, H., Li, X., Fan, H., Cai, C., Liu, B., Fang, Q.(2025) Structure 33: 1844
- PubMed: 40885197
- DOI: https://doi.org/10.1016/j.str.2025.08.004
- Primary Citation of Related Structures:
9VEL - PubMed Abstract:
SPO1-related bacteriophages are promising candidates for phage therapy. We present the 3.0 Å cryo-electron microscopy (cryo-EM) structure of the SPO1 capsid with a triangulation number T = 16, enabling the construction of an atomic model comprising the major capsid protein and three types of minor capsid proteins: gp29.2, gp2.7, and gp36.3. These minor capsid proteins adopt novel folds. They might stabilize the capsid and determine its curvature. Gp29.2 monomers contain a three-blade propeller fold and are located at the 3-fold and quasi-three-fold axes. Gp2.7 forms pentamers atop pentameric capsomers, while gp36.3 binds to the capsid's inner surface, forming star-shaped structures increasing connections between pentameric and hexameric capsomers. The surface exposed regions of gp29.2 and gp2.7 make SPO1 of interest as a nanocage for phage display. Our findings advance the understanding of capsid architecture, stabilization, and local curvature determination for SPO1-related bacteriophages.
- School of Public Health (Shenzhen), Shenzhen Campus of Sun Yat-sen University, No. 66, Gongchang Road, Guangming District, Shenzhen, Guangdong 518107, China.
Organizational Affiliation: