Structural mechanisms of pump assembly and drug transport in the AcrAB-TolC efflux system
Ge, X., Gu, Z., Wang, J.(2025) Elife
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Outer membrane protein TolC | A [auth C1], B [auth C2], C [auth C3] | 493 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: tolC, colE1-i, mtcB, mukA, refI, toc, weeA, b3035, JW5503 | 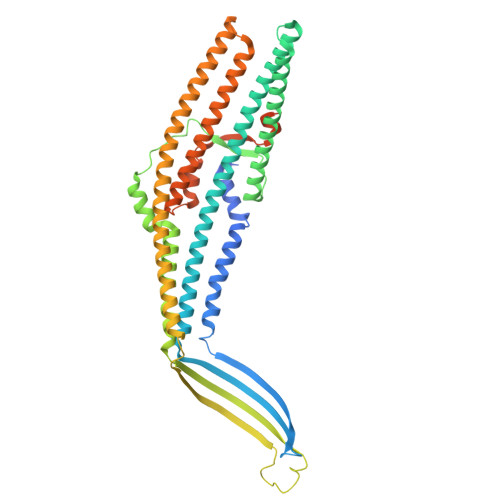 |
UniProt | |||||
Find proteins for P02930 (Escherichia coli (strain K12)) Explore P02930 Go to UniProtKB: P02930 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P02930 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Uncharacterized lipoprotein YbjP | D [auth P1], E [auth P2], F [auth P3] | 171 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: ybjP, b0865, JW0849 |  |
UniProt | |||||
Find proteins for P75818 (Escherichia coli (strain K12)) Explore P75818 Go to UniProtKB: P75818 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P75818 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Multidrug efflux pump subunit AcrA | 397 | Escherichia coli K-12 | Mutation(s): 0 Gene Names: acrA, lir, mtcA, b0463, JW0452 | 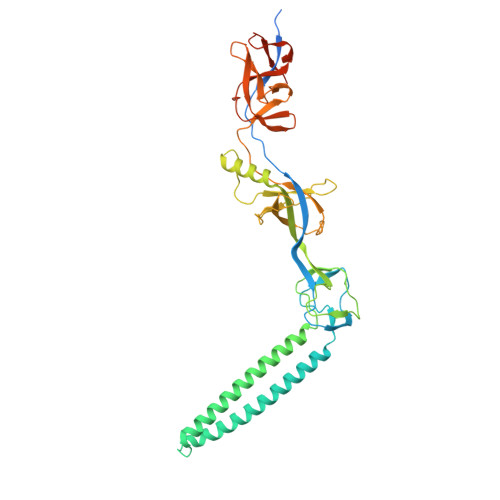 | |
UniProt | |||||
Find proteins for P0AE06 (Escherichia coli (strain K12)) Explore P0AE06 Go to UniProtKB: P0AE06 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0AE06 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Natural Science Foundation of China (NSFC) | China | 32371254 |
| National Natural Science Foundation of China (NSFC) | China | 32171190 |