Bipartite Genetically Encoded Biosensors to Sense Calcium Ion Dynamics at Membrane-Membrane Contact Sites.
Yamaguchi, I., Barazzuol, L., Dematteis, G., Zhu, W., Wen, Y., Drobizhev, M., Lim, D., Campbell, R.E., Cali, T., Nasu, Y.(2025) Anal Chem 97: 19848-19861
- PubMed: 40888292
- DOI: https://doi.org/10.1021/acs.analchem.5c03831
- Primary Citation of Related Structures:
9U9D - PubMed Abstract:
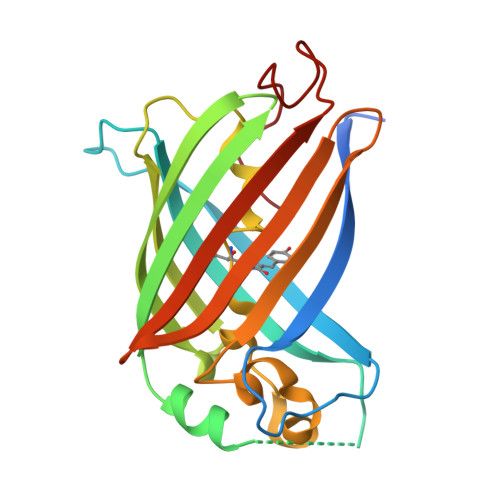
Self-complementing bipartite fluorescent proteins (FPs) are useful tools for the detection of protein-protein proximity and for localizing fluorophores to membrane-membrane contact sites. Here, we report versions of circularly permuted green FP (GFP), red FP (RFP), and mNeonGreen (NG), which are split into a large fragment composed of nine β-strands and a small fragment composed of two β-strands. In each case, the large and small fragments can associate in live cells to form the complete 11-stranded FP β-barrel. We further converted each of these three self-complementing FPs into bipartite calcium ion (Ca 2+ ) biosensors. We demonstrate that appropriately targeted versions of these split FPs, and split FP-based biosensors, can be functionally assembled at membrane-membrane contact sites. We employ the bipartite NG-based Ca 2+ biosensor for visualization of pharmacologically induced Ca 2+ release at mitochondria-endoplasmic reticulum contact sites (MERCs).
- Department of Chemistry, School of Science, The University of Tokyo, Bunkyo-ku, Tokyo113-0033, Japan.
Organizational Affiliation: