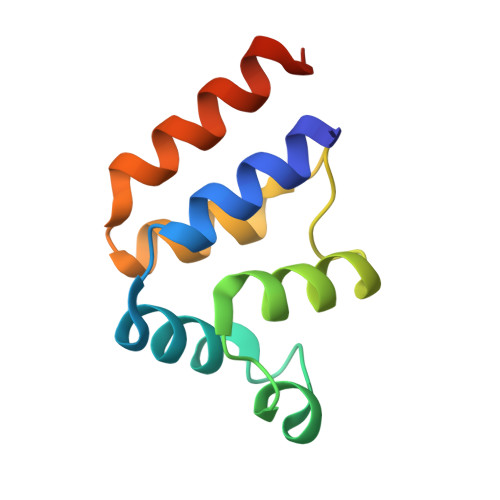
FADD DED filaments coordinate complex IIa assembly during TNF-induced apoptosis.
Chen, Y., Huynh, V.T., Lai, L., Liu, P., Li, T., Tan, Y.B., Chew, C.S., Velazquez, A.M.V., Samsudin, F., Marzinek, J.K., Bond, P.J., Wu, B., Luo, D., Tergaonkar, V.(2025) Proc Natl Acad Sci U S A 122: e2425802122-e2425802122
- PubMed: 40838879
- DOI: https://doi.org/10.1073/pnas.2425802122
- Primary Citation of Related Structures:
9U6E, 9U7A - PubMed Abstract:
Extrinsic apoptosis is initiated by signaling from death receptors, leading to the assembly of RIPK1, FADD, and caspase-8 complex. Subsequently, caspase-8 forms a filamentous structure through the oligomerization of its tandem death effector domain (tDED), resulting in caspase activation and cell death. Although the DED of FADD ( FADD DED) is homologous to the tDEDs of caspase-8 ( casp8 tDED) and both oligomerize to function, the functional form of FADD DED oligomer in extrinsic apoptosis remains unclear. Here, using cryogenic-electron microscopy, we elucidate the structure of FADD DED filaments comprising three helical chains assembled through three types of iterative interactions. Mutations disrupting FADD DED filament formation impair the recruitment of RIPK1 and caspase-8, and abrogate the cell death response, suggesting that FADD DED filamentation represents an important mechanistic step in the initiation of TNF-induced extrinsic apoptosis. Contrary to the belief that the homotypic death domains of RIPK1 and FADD are solely responsible for their interaction, we here show this interaction requires FADD DED filamentation. Furthermore, cFLIP can disrupt FADD DED filaments, uncovering an additional antiapoptotic mechanism of cFLIP beyond its disruption of caspase-8 filament. Molecular dynamics simulations reveal that FADD DED filament thermodynamically favors casp8 tDED monomer over FADD DED monomer, thus explaining the hierarchy and stoichiometry of FADD/caspase-8 complex assembly. These findings highlight the hitherto unappreciated roles of FADD DED filament formation in extrinsic apoptosis.
- Laboratory of NF-κB Signalling, Institute of Molecular and Cell Biology, Agency for Science, Technology and Research, Singapore 138673, Singapore.
Organizational Affiliation: