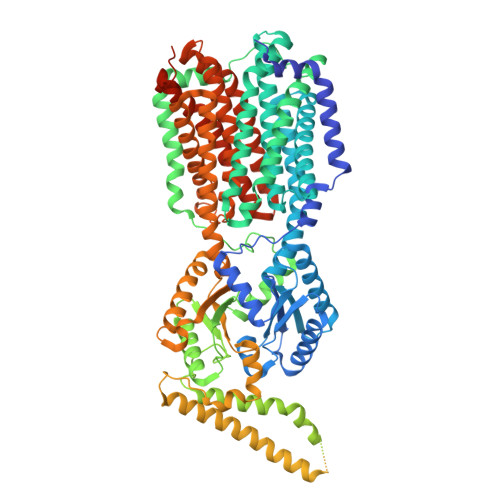
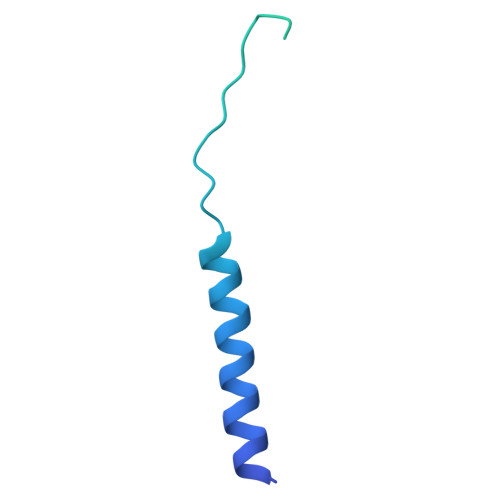
Structural elucidation of the hexameric MmpS4-MmpL4 complex from Mycobacterium tuberculosis.
Earp, J.C., Lichti, N.P., Garaeva, A.A., Meikle, V., Niederweis, M., Seeger, M.A.(2026) bioRxiv
- PubMed: 41542548
- DOI: https://doi.org/10.64898/2026.01.07.698164
- Primary Citation of Related Structures:
9SYJ, 9SYT, 9SYV - PubMed Abstract:
Mycobacterium tuberculosis contains thirteen Mycobacterial membrane protein Large (MmpL) transporters, which belong to the family of secondary active RND transporters. MmpL4 and MmpL5, together with their operon partners MmpS4 and MmpS5, export the mycobacterial siderophore mycobactin and the last resort TB drug bedaquiline. Recently, we determined a structure of the MmpL4 monomer in complex with desferrated mycobactin, which lacked a functionally essential coiled-coil domain predicted to extend far into the periplasm. Here, we present a cryo-EM structure of the hexameric (MmpS4) 3 -(MmpL4) 3 complex, which was enabled by rational disulfide cross-links based on AlphaFold predictions. We observed density for the coiled-coil domain, which protrudes into the periplasmic space at an angle of around 60° relative to the symmetry axis of the MmpL4 trimer. In the context of the hexameric complex, MmpL4's conformation differs strikingly from the one observed for monomeric MmpL4, which includes formation of a large cavity in the periplasmic domain and rearrangements of conserved proton coupling residues at the transmembrane domain. Our work provides an experimental workflow to obtain single particle cryo-EM structures of labile multiprotein complexes by AlphaFold-informed stabilization of predicted protein interfaces.
- Institute of Medical Microbiology, University of Zurich, Zurich, Switzerland.
Organizational Affiliation: