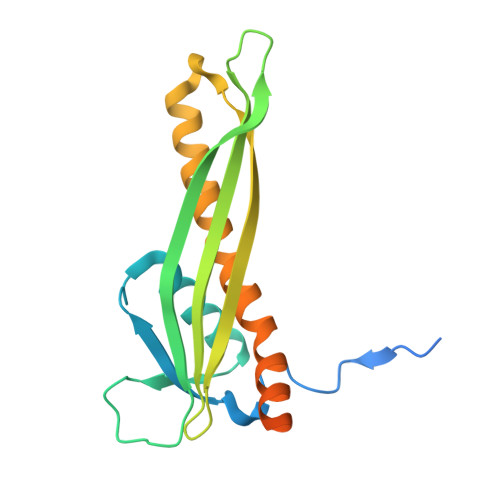
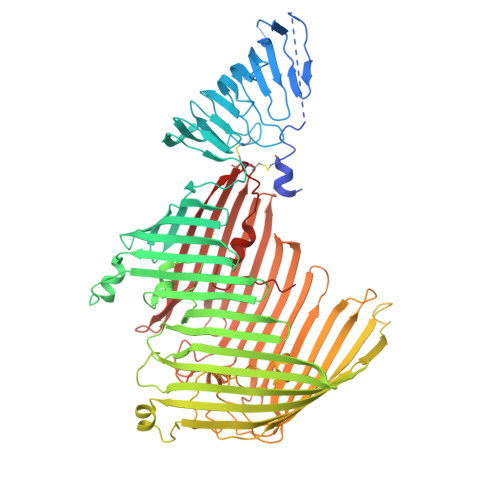
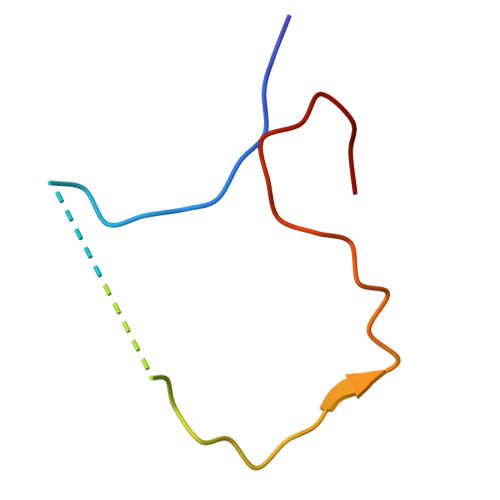
Small siphophage binding to an open state of the LptDE outer membrane lipopolysaccharide translocon.
Dunbar, E., Clark, R., Basle, A., Allyjaun, S., Newman, H., Hubbard, J., Khalid, S., van den Berg, B.(2025) Proc Natl Acad Sci U S A 122: e2516650122-e2516650122
- PubMed: 41296721
- DOI: https://doi.org/10.1073/pnas.2516650122
- Primary Citation of Related Structures:
9RPR, 9RPS, 9RPT, 9RPW, 9RQI - PubMed Abstract:
Bacteriophages are bacterial viruses that provide alternatives to small-molecule drugs to combat infections by antibiotic-resistant bacteria. To infect a bacterial host, a phage needs to bind to the bacterial surface via receptor binding proteins (RBPs), which are critical for determining host specificity. For functionally important receptors, the RBP-receptor interaction could be exploited via phage steering, where emerging bacterial resistance due to receptor modification could make bacteria less fit or virulent. Despite this, relatively little is known about RBP-receptor interactions. Here, we build on the recent discovery of coliphages that have the outer membrane (OM) lipopolysaccharide translocon LptDE as their terminal receptor and show via cryogenic electron microscopy that, surprisingly, the RBP of the small siphophage Oekolampad binds to a hitherto unobserved, open state of LptDE. The open lateral gate of LptD is occupied by a β-strand peptide originating from the degraded N-terminal jellyroll domain of LptD, suggesting the possibility of LptD inhibition via peptidomimetics. A structure of LptDE in complex with the superinfection exclusion (SE) protein Rtp45 of the Oekolampad-related phage Rtp shows a mechanism of SE where Rtp45-induced conformational changes in LptD resulting from steric clashes preclude RBP binding. Finally, analysis of spontaneous Oekolampad-resistant Escherichia coli mutants identifies mutations in LptD that abolish the LptDE-RBP interaction in vitro. SDS-EDTA sensitivity assays of the mutants show no major OM defects, consistent with largely preserved LptDE function, and suggesting that phage steering via LptDE might be challenging.
- Biosciences Institute, The Medical School, Newcastle University, Newcastle upon Tyne NE2 4HH, United Kingdom.
Organizational Affiliation: