Structural mechanism of the minimal ligase HECTD3
Huber, J., Esposito, D., Maslen, S., Rittinger, K.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| E3 ubiquitin-protein ligase HECTD3 | 864 | Homo sapiens | Mutation(s): 0 Gene Names: HECTD3 EC: 2.3.2.26 | 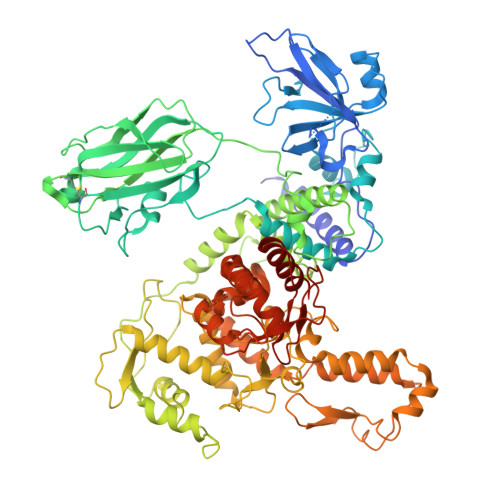 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q5T447 (Homo sapiens) Explore Q5T447 Go to UniProtKB: Q5T447 | |||||
PHAROS: Q5T447 GTEx: ENSG00000126107 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q5T447 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | |
| RECONSTRUCTION | cryoSPARC |
| Funding Organization | Location | Grant Number |
|---|---|---|
| The Francis Crick Institute | United Kingdom | CC2075, CC2000 |
| UK Research and Innovation (UKRI) | United Kingdom | CC2075, CC2000 |
| Cancer Research UK | United Kingdom | CC2075, CC2000 |
| Wellcome Trust | United Kingdom | CC2075, CC2000 |