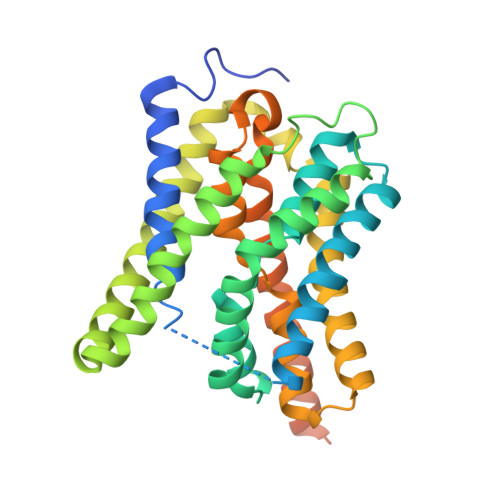
Structure of nanobody-inhibited state of human bile salt transporter NTCP.
Yoon, D., Nosol, K., Rasouli, A., Bang-Sorensen, R., Irobalieva, R.N., Liu, H., Tajkhorshid, E., Locher, K.P.(2026) Structure 34: 113-122.e7
- PubMed: 41138719
- DOI: https://doi.org/10.1016/j.str.2025.09.012
- Primary Citation of Related Structures:
9QZQ - PubMed Abstract:
Sodium-taurocholate co-transporting polypeptide (NTCP) is a sodium-dependent transporter mediating the hepatic uptake of bile salts and serving as the receptor of hepatitis B and D viruses. While previous studies identified binding sites for sodium ions and substrates, the mechanism remains controversial. We here report a high-resolution structure of NTCP in a closed-tunnel conformation that does not feature substrate binding sites but reveals evidence of two bound sodium ions. To evaluate the functional relevance of this state and gain insight into the transport mechanism, we performed μs-scale molecular dynamics simulations of NTCP starting from distinct conformations and substrate and ion configurations. We observed that both the closed-tunnel and open-tunnel conformations are highly stable, but that the sodium ions and bile salt molecules can shift positions without substantial conformational changes. Our results suggest that the closed-tunnel conformation might represents an inactive state rather than an essential component of a productive transport cycle.
- Institute of Molecular Biology and Biophysics, ETH Zürich, 8093 Zürich, Switzerland.
Organizational Affiliation: