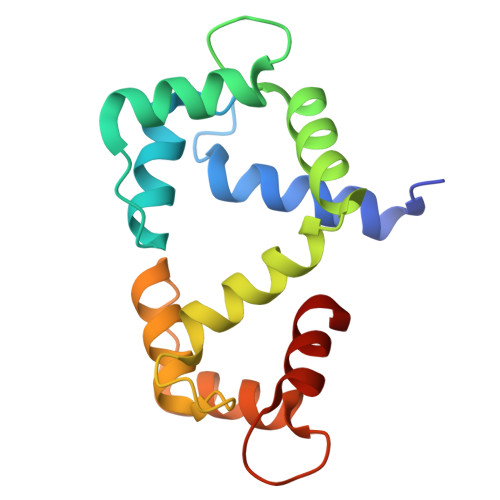
Structural Basis and Functional Analysis of NMDA Receptor Regulation by Calmodulin.
Bej, A., Erickson-Oberg, M.Q., Nigam, A., Yu, I., Hell, J.W., Johnson, J.W., Ames, J.B.(2026) J Biological Chem : 111131-111131
- PubMed: 41513089
- DOI: https://doi.org/10.1016/j.jbc.2026.111131
- Primary Citation of Related Structures:
9PQH, 9PQI - PubMed Abstract:
The synaptic plasticity mechanisms that are thought to underlie learning and memory require Ca 2+ influx mediated by N-methyl-D-aspartate receptors (NMDARs) composed of glycine-binding GluN1 and glutamate-binding GluN2 subunits. Calmodulin (CaM) binding to the cytosolic regions in both GluN1 (residues 841-865, called GluN1-C0) and GluN2A (residues 1004-1023, called GluN2A-C0) may be important for Ca 2+ -dependent channel desensitization (CDD). Here, we report NMR, ITC and electrophysiological experiments to probe the structure and functional role of Ca 2+ -bound CaM (Ca 2+ -CaM) binding to both GluN1 and GluN2A subunits. Our ITC studies show that the GluN1-C0 peptide binds to both the N-lobe and C-lobe of Ca 2+ -CaM, whereas the GluN2A-C0 peptide binds to only the Ca 2+ -CaM C-lobe. Our NMR analysis reveals GluN2A residues (W1014 and V1018) interact with exposed hydrophobic residues in the Ca 2+ -CaM C-lobe. The NMR structure of Ca 2+ -CaM bound to the GluN1-C0 peptide indicates the two CaM lobes bind to opposite sides of the GluN1-C0 helix (C-lobe contacts M848, F852, A853 and N-lobe contacts A854, V855, W858). The GluN1 mutant F852E and the GluN2A mutant W1014E both perturbed CaM binding in ITC studies, and also diminished electrophysiologically-measured CDD, suggesting CaM interaction with these residues contributes to CDD. We propose a structural mechanism of CDD wherein channel desensitization is caused by the binding of four CaM per NMDAR subunit tetramer.
- Department of Chemistry, University of California, Davis, CA; Department of Pharmacology, University of California, Davis, CA.
Organizational Affiliation: