High-Affinity, Structure-Validated and Selective Macrocyclic Peptide Tools for Chemical Biology Studies of Huntingtin.
Wolf, E., Fanti, R., Ikenoue, T., Deme, J.C., Balakrishnan, S., Keith, B.A., Alteen, M.G., Chandrasekaran, R., Yadav, M., Bhajiawala, R., Ackloo, S., Feng, J., Pouladi, M.A., Edwards, A.M., Wilson, D., Lea, S.M., Suga, H., Harding, R.J.(2025) bioRxiv
- PubMed: 41030958
- DOI: https://doi.org/10.1101/2025.08.06.668955
- Primary Citation of Related Structures:
9PMW, 9PN0 - PubMed Abstract:
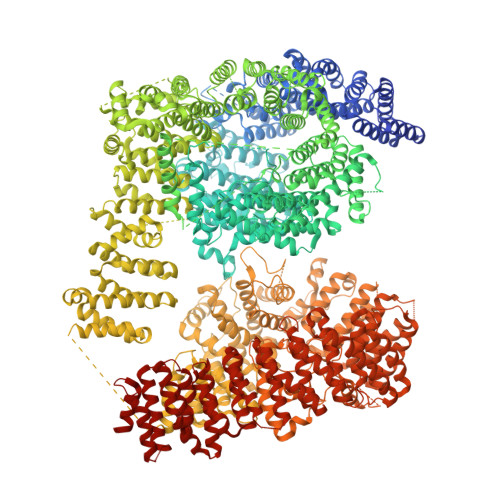
Huntington's disease (HD) is a fatal neurodegenerative disorder caused by a CAG repeat expansion in the Huntingtin ( HTT ) gene, with no disease-modifying therapies currently available. The precise molecular function of the HTT protein is unclear, and the lack of selective chemical tools has limited functional studies. We have identified and characterized macrocyclic peptide binders targeting HTT. These binders exhibit low-nanomolar affinity in vitro and engage distinct HTT and HTT-HAP40 interfaces, as revealed by hydrogen-deuterium exchange mass spectrometry and cryo-electron microscopy. Chemoproteomics confirmed selective binding in cell extracts from wildtype but not HTT-null cell lines. HAP40 consistently and stoichiometrically co-purified with HTT across cell lines, including with HTT variants containing different CAG repeat lengths, highlighting the broad presence of the HTT-HAP40 complex.
- Structural Genomics Consortium, University of Toronto, Toronto, ON M5G 1L7, Canada.
Organizational Affiliation: