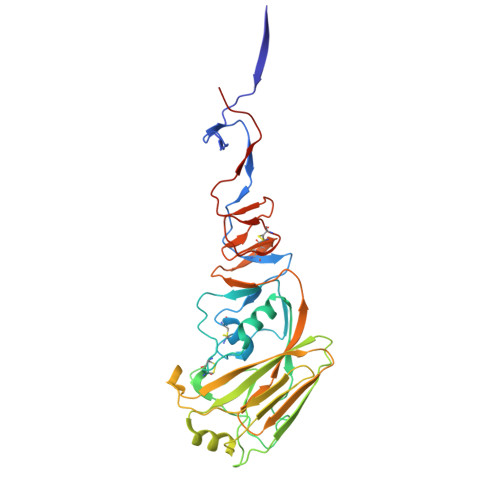
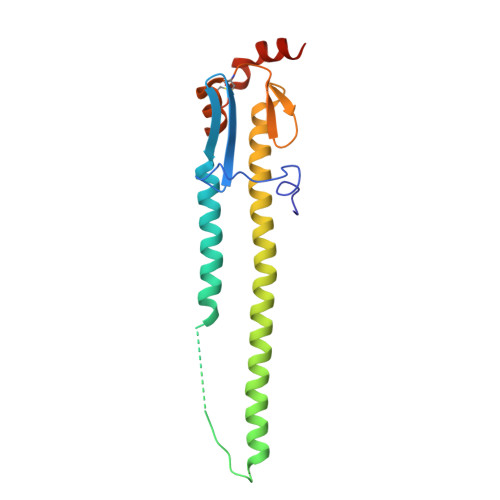
V H H antibody loop guides design of a synthetic macrocyclic peptide that potently blocks influenza virus membrane fusion.
Kadam, R.U., Juraszek, J., Brandenburg, B., Garg, D., Zhu, X., Jongeneelen, M., Schepens, W.B.G., Stoops, B., Vermond, J., Goutier, W., Tang, C., Blokland, S., Vogels, R., Friesen, R.H.E., van Dongen, M.J.P., Wilson, I.A.(2025) Npj Viruses 3: 83-83
- PubMed: 41413306
- DOI: https://doi.org/10.1038/s44298-025-00166-1
- Primary Citation of Related Structures:
9PHD, 9PHQ, 9PHS, 9PHT - PubMed Abstract:
Miniaturizing biologically complex structural motifs to produce synthetic functional mimetics holds significant promise for development of new therapeutic modalities. Here, we demonstrate a unique approach using the key binding loop of the single variable domain of a heavy chain (V H H) llama antibody as a starting point for peptide design. V H H antibodies of camelids and sharks generally have longer, but more ligand-efficient complementarity determining region 3 (CDR3) loops and are relatively stable structures. We harnessed these attributes as templates for design of a series of synthetic macrocyclic peptides. The designed peptides exhibit nanomolar binding to influenza hemagglutinin (HA) and heterosubtypic in vitro neutralization breadth against influenza A viruses by inhibiting the low pH mediated HA conformational changes that lead to membrane fusion. X-ray structures of peptide-HA complexes reveal high structural mimicry with the parent V H H antibody. One such macrocycle peptide candidate is promising for further development of broad protection against influenza A group 1 viruses.
- Department of Integrative Structural and Computational Biology, The Scripps Research Institute, La Jolla, CA, USA. rkadam3@its.jnj.com.
Organizational Affiliation: