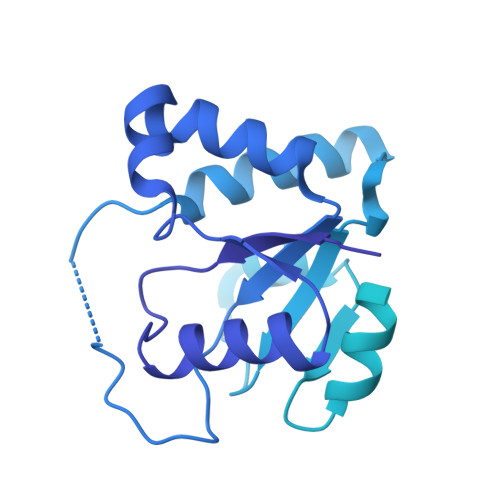
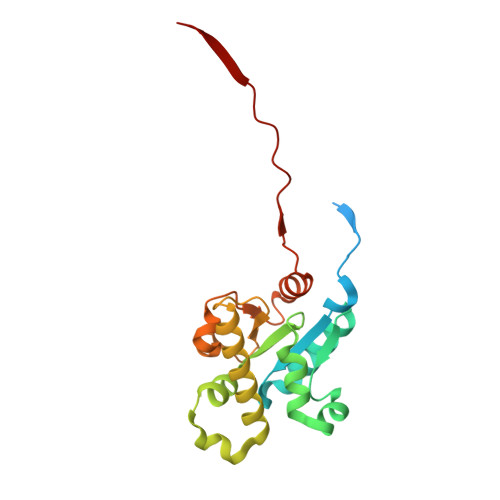
Structural basis for ACT1 oligomerization induced by IL-17 receptor hetero-tetramer.
Zhang, H., Bai, X.C., Zhang, X.(2025) Nat Commun 17: 836-836
- PubMed: 41397964
- DOI: https://doi.org/10.1038/s41467-025-67536-4
- Primary Citation of Related Structures:
9OT1 - PubMed Abstract:
The IL-17 receptors (IL-17Rs) play critical roles in immunity and inflammatory diseases. IL-17-induced heteromeric complexes between IL-17RA and another IL-17R trigger signaling by binding the downstream transducer ACT1 through interactions between their intracellular SEF/IL-17R (SEFIR) domains. The molecular mechanism of this process remains unclear. Here we present the cryo-EM structure of the complex of IL-17RA, IL-17RB and ACT1, showing that the IL-17RA and IL-17RB SEFIR domains form an asymmetric hetero-tetramer. The two IL-17RA SEFIR domains serve as the base to recruit ACT1, while IL-17RB stabilizes the IL-17RA dimer but makes no interaction with ACT1. IL-17RB, IL-17RA, and multiple ACT1 together form a double-stranded helical assembly. The C-terminal SEFIR extension (SEFEX) of IL-17RA acts as a molecular tendril to help anchor the ACT1 protomers. The structural model is supported by our mutational analyses. These findings reveal the basis for the formation for the signalosome of the IL-17 receptors and ACT1 critical for immune signaling.
- Department of Pharmacology, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Organizational Affiliation: