Autoinhibited BRAF:(14-3-3)2:MEK complex from Insect cells
Martinez Fiesco, J.A., Zhang, P.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Serine/threonine-protein kinase B-raf | 766 | Homo sapiens | Mutation(s): 0 Gene Names: BRAF, BRAF1, RAFB1 EC: 2.7.11.1 | 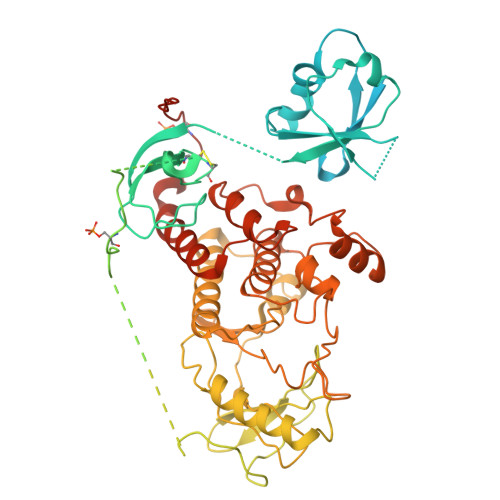 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P15056 (Homo sapiens) Explore P15056 Go to UniProtKB: P15056 | |||||
PHAROS: P15056 GTEx: ENSG00000157764 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P15056 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Dual specificity mitogen-activated protein kinase kinase 1 | 393 | Homo sapiens | Mutation(s): 0 Gene Names: MAP2K1, MEK1, PRKMK1 EC: 2.7.12.2 | 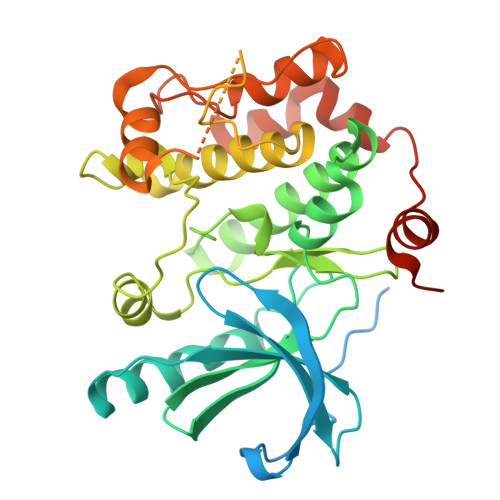 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q02750 (Homo sapiens) Explore Q02750 Go to UniProtKB: Q02750 | |||||
PHAROS: Q02750 GTEx: ENSG00000169032 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q02750 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| 14-3-3 protein zeta/delta | 245 | Homo sapiens | Mutation(s): 0 Gene Names: YWHAZ | 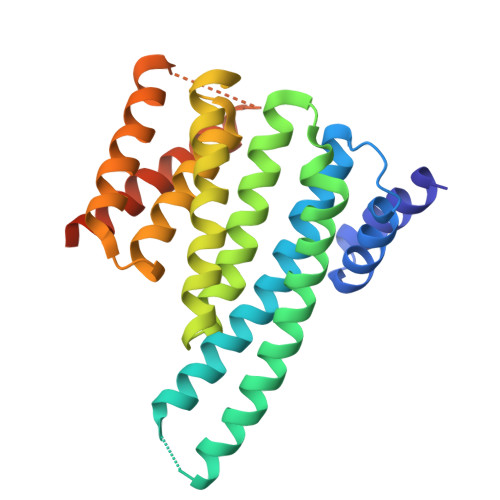 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P63104 (Homo sapiens) Explore P63104 Go to UniProtKB: P63104 | |||||
PHAROS: P63104 GTEx: ENSG00000164924 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P63104 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| CHU (Subject of Investigation/LOI) Query on CHU | G [auth B] | N-(3-fluoro-4-{[4-methyl-2-oxo-7-(pyrimidin-2-yloxy)-2H-chromen-3-yl]methyl}pyridin-2-yl)-N'-methylsulfuric diamide C21 H18 F N5 O5 S LMMJFBMMJUMSJS-UHFFFAOYSA-N |  | ||
| ZN Query on ZN | E [auth A], F [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| SEP Query on SEP | A | L-PEPTIDE LINKING | C3 H8 N O6 P |  | SER |
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.21.1_5286 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Cancer Institute (NIH/NCI) | United States | -- |