CN: TAK1 complex explains CN substrate specifity
Shirakawa, K.T., Parikh, T., Page, R., Peti, W.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Protein phosphatase 3 catalytic subunit alpha | 370 | Homo sapiens | Mutation(s): 1 Gene Names: PPP3CA, CALNA, CNA EC: 3.1.3.16 | 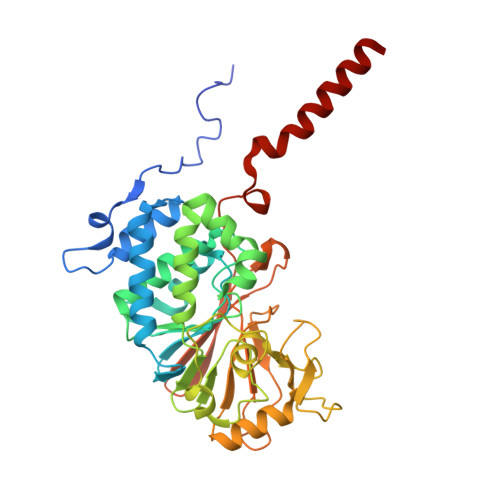 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q08209 (Homo sapiens) Explore Q08209 Go to UniProtKB: Q08209 | |||||
PHAROS: Q08209 GTEx: ENSG00000138814 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q08209 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Calcineurin subunit B type 1 | 156 | Homo sapiens | Mutation(s): 0 Gene Names: PPP3R1, CNA2, CNB | 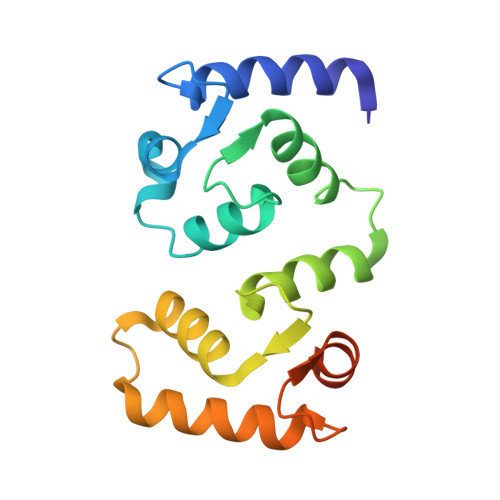 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P63098 (Homo sapiens) Explore P63098 Go to UniProtKB: P63098 | |||||
PHAROS: P63098 GTEx: ENSG00000221823 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P63098 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Sodium/hydrogen exchanger 1 | 48 | Homo sapiens | Mutation(s): 0 Gene Names: SLC9A1, APNH1, NHE1 | 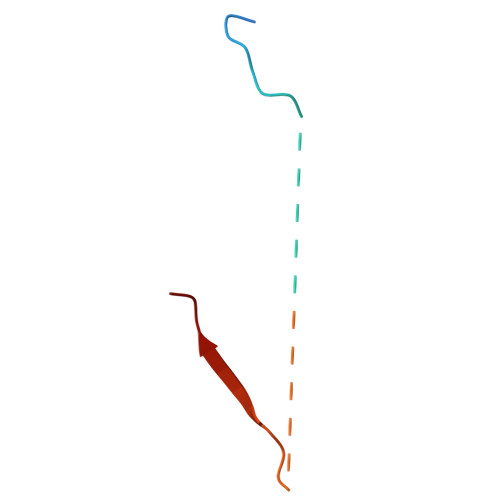 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P19634 (Homo sapiens) Explore P19634 Go to UniProtKB: P19634 | |||||
PHAROS: P19634 GTEx: ENSG00000090020 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P19634 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 4 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| PEG Query on PEG | D [auth A] | DI(HYDROXYETHYL)ETHER C4 H10 O3 MTHSVFCYNBDYFN-UHFFFAOYSA-N |  | ||
| ZN Query on ZN | F [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| FE Query on FE | E [auth A] | FE (III) ION Fe VTLYFUHAOXGGBS-UHFFFAOYSA-N |  | ||
| CA Query on CA | G [auth B], H [auth B], I [auth B], J [auth B] | CALCIUM ION Ca BHPQYMZQTOCNFJ-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 79.49 | α = 90 |
| b = 126.501 | β = 90 |
| c = 127.657 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| autoPROC | data reduction |
| autoPROC | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R01GM144379 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | R01NS124666 |