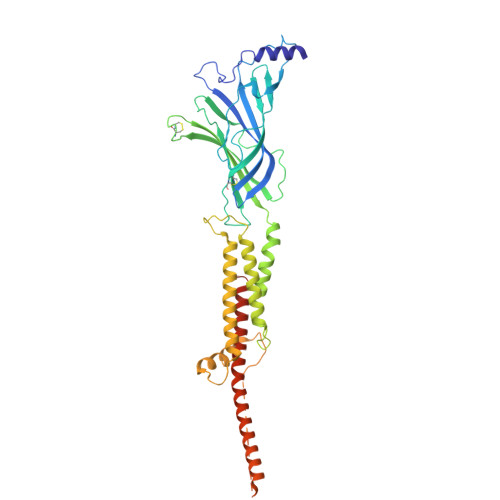
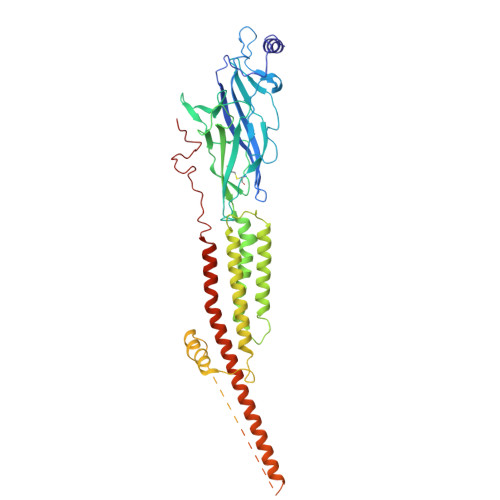
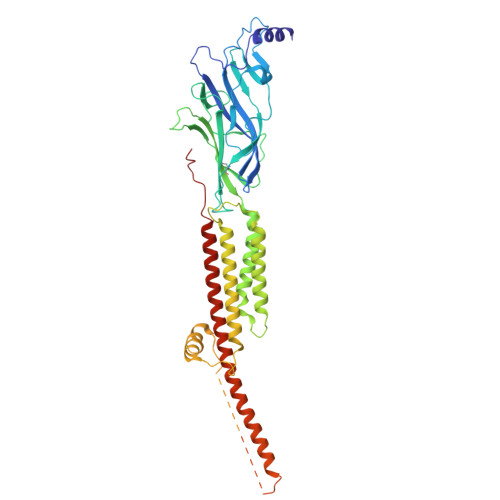
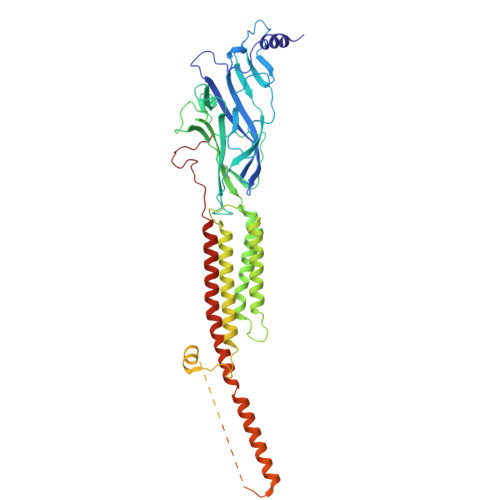
Shape-shifting conotoxins reveal divergent pore-targeting mechanisms in nicotinic receptors.
Bhattacharjee, B., Noviello, C.M., Rahman, M.M., Mayer, J.P., Gajewiak, J., McIntosh, J.M., Hibbs, R.E., Stowell, M.H.B.(2025) Structure
- PubMed: 41468893
- DOI: https://doi.org/10.1016/j.str.2025.12.003
- Primary Citation of Related Structures:
9NX0, 9NX1, 9NX2 - PubMed Abstract:
The neuronal α7 nicotinic acetylcholine receptor (α7-nAChR) and muscle-type nicotinic acetylcholine receptor (mt-nAChR) are pivotal in synaptic signaling within the brain and the neuromuscular junction respectively. Additionally, they are both targets of a wide range of drugs and toxins. Here, we utilize cryo-EM to delineate structures of these nAChRs in complex with the conotoxins ImI and ImII from Conus imperialis. Despite nominal sequence differences, ImI and ImII exhibit discrete binding preferences and adopt drastically different conformational states upon binding. ImI engages the orthosteric sites of α7-nAChR, while ImII forms distinct pore-bound complexes with both α7-nAChR and mt-nAChR. Strikingly, ImII adopts a compact globular conformation that binds as a monomer to the α7-nAChR pore and as an oblate dimer to the mt-nAChR pore. These structures advance our understanding of nAChR-ligand interactions and the subtle sequence variations that result in dramatically altered functional outcomes in small peptide toxins.
- Department of Molecular, Cellular & Developmental Biology, University of Colorado, Boulder, CO, USA.
Organizational Affiliation: