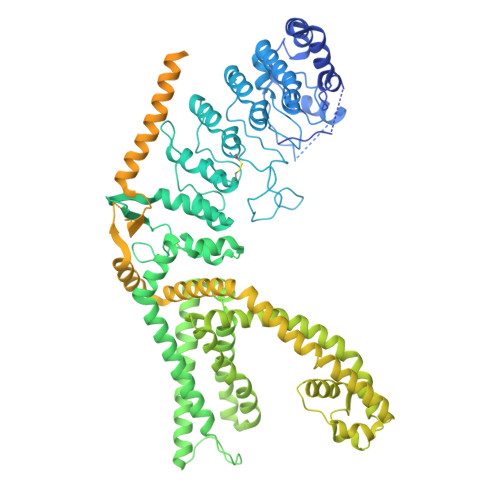
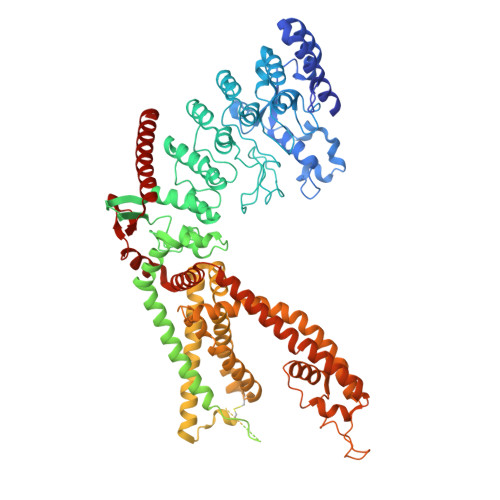
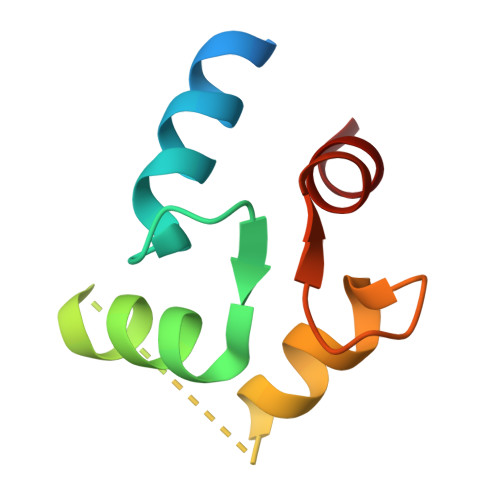
Visualizing insecticide control of insect TRP channel function and assembly.
Fedor, J.G., Kandasamy, R., Park, C.G., Suo, Y., Weisel, M., Rankl, N.B., Nesterov, A., Lee, S.Y.(2025) Nat Commun 17: 595-595
- PubMed: 41392056
- DOI: https://doi.org/10.1038/s41467-025-67287-2
- Primary Citation of Related Structures:
9NVN, 9NVO, 9NVP, 9NVQ, 9NVR, 9NVS - PubMed Abstract:
Insecticides are vital to combating world food shortages and transmission of vector-borne human diseases. Increasing insecticide resistance necessitates discovery of novel compounds against underutilized targets. Nanchung (Nan) and Inactive (Iav), the transient receptor potential vanilloid-type (TRPV) channels in insects, likely form a heteromeric channel (Nan-Iav) and are localized in mechanosensory chordotonal organs which confer gravitaxis, hearing and proprioception. Several insecticides, such as afidopyropen (AP), target Nan-Iav through unknown mechanisms. Effective against piercing-sucking (hemipteran) insects, AP disrupts chordotonal functions preventing feeding. AP can bind to Nan alone, but only Nan-Iav exhibits channel activity with agonists including endogenous nicotinamide (NAM). Despite its importance as an insecticide target, much is unknown about Nan-Iav, such as channel assembly, modulator binding sites, and Ca 2+ -dependent regulation, hampering further insecticide development. Here we present the cryo-electron microscopy structures of hemipteran Nan-Iav with calmodulin bound in the apo state and with AP and NAM bound to cytosolic ankyrin repeat domain (ARD) interfaces. Unexpectedly, we found that Nan alone can form a pentamer, stabilized through AP-mediated ARD interactions. Our study provides molecular insights into insecticide and agonist interactions with Nan-Iav, highlighting the importance of the ARD on channel function and assembly, while also probing regulation by Ca 2+ .
- Department of Biochemistry, Duke University School of Medicine, Durham, NC, USA.
Organizational Affiliation: