An evolutionarily conserved salt bridge stabilizes the active site for GTP hydrolysis in Rho GTPases
Marcus, K., Schwabe, M., Knihtila, R., Mattos, C.(null) J Biological Chem
Experimental Data Snapshot
Starting Model: experimental
View more details
(null) J Biological Chem
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Transforming protein RhoA | 181 | Homo sapiens | Mutation(s): 1 Gene Names: RHOA, ARH12, ARHA, RHO12 EC: 3.6.5.2 | 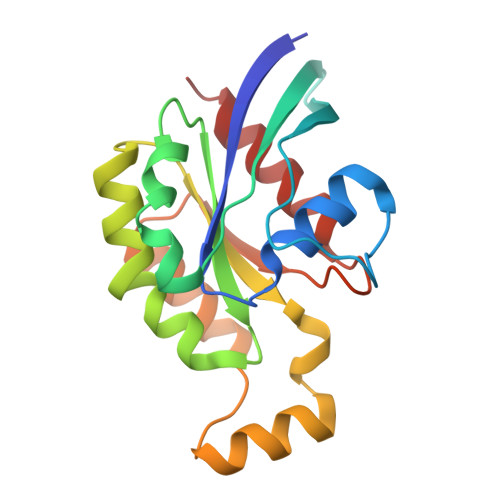 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P61586 (Homo sapiens) Explore P61586 Go to UniProtKB: P61586 | |||||
PHAROS: P61586 GTEx: ENSG00000067560 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P61586 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 5 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| GSP Query on GSP | E [auth A], KA [auth C], T [auth B], TA [auth D] | 5'-GUANOSINE-DIPHOSPHATE-MONOTHIOPHOSPHATE C10 H16 N5 O13 P3 S XOFLBQFBSOEHOG-UUOKFMHZSA-N |  | ||
| IOD Query on IOD | AA [auth B] AB [auth D] BA [auth B] BB [auth D] CA [auth B] | IODIDE ION I XMBWDFGMSWQBCA-UHFFFAOYSA-M |  | ||
| CL Query on CL | HA [auth B] HB [auth D] IA [auth B] JA [auth B] L [auth A] | CHLORIDE ION Cl VEXZGXHMUGYJMC-UHFFFAOYSA-M |  | ||
| MG Query on MG | F [auth A], LA [auth C], MA [auth C], U [auth B], UA [auth D] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| NA Query on NA | Y [auth B] | SODIUM ION Na FKNQFGJONOIPTF-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 54.217 | α = 67.842 |
| b = 60.532 | β = 78.049 |
| c = 67.134 | γ = 68.362 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| PHENIX | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Science Foundation (NSF, United States) | United States | 1517295 |