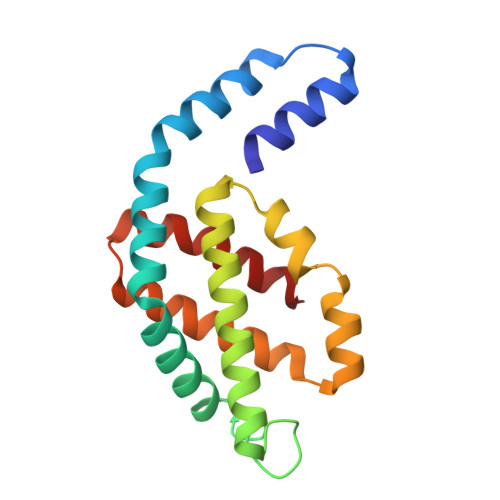
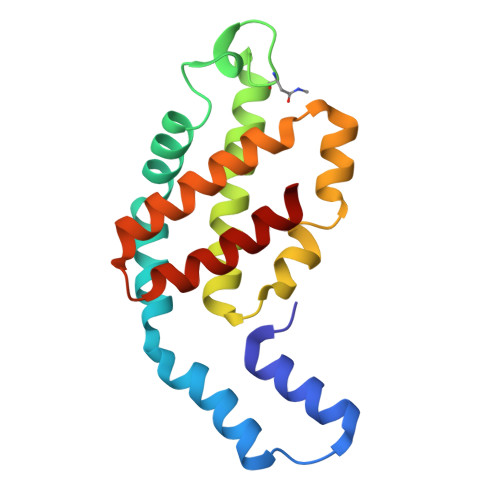
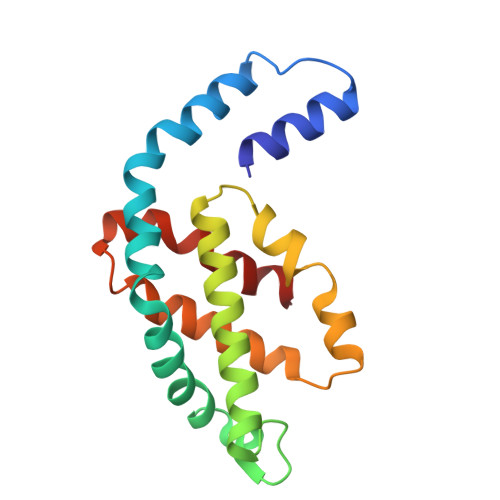
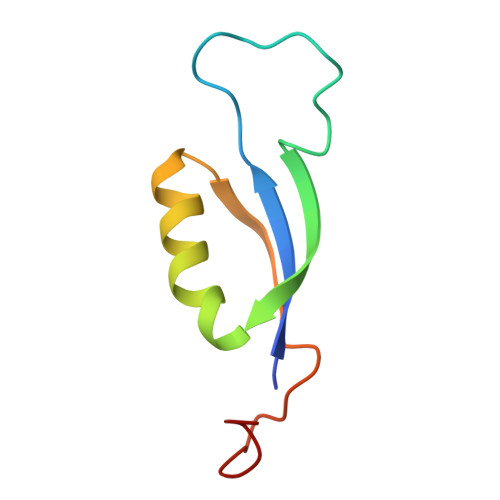
Light-induced structural adaptation of the bundle-shaped phycobilisome from thylakoid-lacking cyanobacterium Gloeobacter violaceus.
Ma, J., You, X., Sun, S., Sui, S.F.(2025) Nat Commun 16: 5956-5956
- PubMed: 40593595
- DOI: https://doi.org/10.1038/s41467-025-60673-w
- Primary Citation of Related Structures:
9K9W, 9M3J - PubMed Abstract:
Gloeobacter diverged from other lineages early in cyanobacterial evolution, preferentially growing under low light intensity conditions. Among cyanobacteria, G. violaceus exhibits unique features, including lack of a thylakoid membrane and bundle-shaped antenna phycobilisomes (PBSs), densely packed and well-organized on the plasma membrane. However, without high-resolution structures, it has remained unclear how G. violaceus PBSs assemble into a bundle-shaped configuration. Here we solve the cryo-EM structures of PBSs from G. violaceus cells cultured under low (Sr-PBS) or moderate (Lr-PBS) light intensity. These structures reveal two unique linker proteins, L RC 91kDa and L RC 81kDa , that play a key role in the PBS architecture. Analysis of the bilin arrangement indicates that the bundle-shaped structure allows efficient energy transfer among rods. Moreover, comparison between Lr-PBS and Sr-PBS uncovers a distinct mode of adaption to increased light intensity wherein the ApcA 2 -ApcB 3 -ApcD layer can be blocked from binding to the core by altering structural elements exclusively found in the G. violaceus L CM . This study illustrates previously unrecognized mechanisms of assembly and adaptation to varying light intensity in the bundle-shaped PBS of G. violaceus.
- State Key Laboratory of Membrane Biology, Beijing Frontier Research Center for Biological Structures, School of Life Sciences, Tsinghua University, Beijing, China. majf@nankai.edu.cn.
Organizational Affiliation: