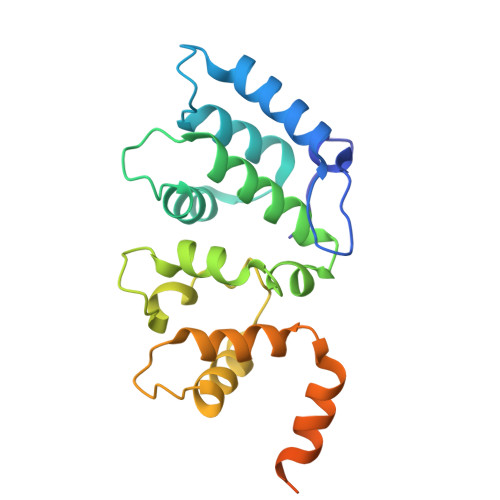
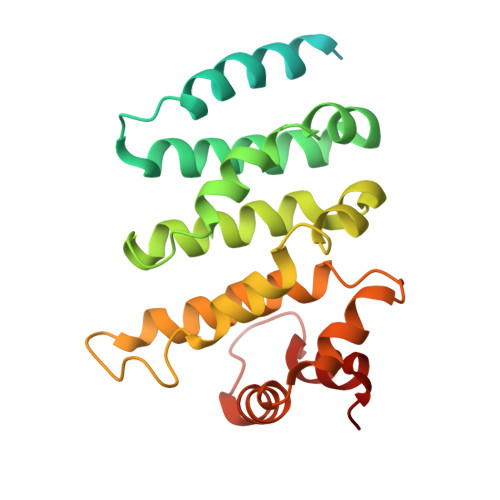
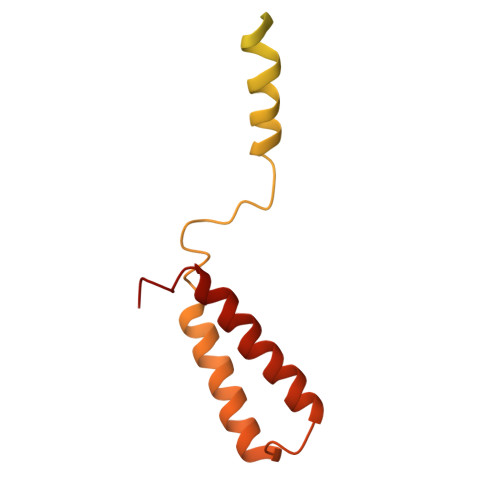
ARMH2 is a cytosolic component of CatSper crucial for sperm function.
Zhao, Q., Lin, S., Kang, H., Ru, Y., Xu, Q., Yu, Z., Huang, X., De Rito, C., Sassi, G., Wang, S., Sun, S., Sun, R., Cheng, H., Zhu, Y., Liu, M., Zhang, Y., Jiang, M., Percudani, R., Chung, J.J., Zeng, X., Yan, Z., Wu, J.(2025) Nat Commun 16: 10243-10243
- PubMed: 41271765
- DOI: https://doi.org/10.1038/s41467-025-65952-0
- Primary Citation of Related Structures:
9LWO - PubMed Abstract:
Sperm capacitation and fertilization are highly regulated by Ca 2+ signaling. CatSper, a sperm-specific calcium channel, plays a crucial role in sperm hyperactivated motility and fertility by mediating Ca 2+ influx into sperm. CatSper is the most complicated ion channel known, comprising the pore-forming CATSPER1-4 and multiple auxiliary subunits. However, our previous structural study of mouse CatSper suggests the presence of potential component(s) that remain to be identified. The identity and functional significance of the missing piece(s) of CatSper remain elusive. Here, by combining cryo-EM, mass spectrometry, AlphaFold structure prediction, and coevolutionary analysis, we identify armadillo-like helical domain containing 2 (ARMH2) as a cytosolic component of CatSper. ARMH2 forms a cytosolic ternary subcomplex with EFCAB9 and CATSPERζ, which contributes to the stable assembly of the linear arrangement of CatSper nanodomains along the sperm tail and regulates the pH and Ca 2+ sensitivity of the channel. Loss of ARMH2 leads to compromised physiological activation of CatSper, thereby resulting in asthenozoospermia and severe subfertility. These findings show that ARMH2 is crucial for sperm function and provide fresh insights into the composition and functional regulation of CatSper. The integrated methodology employed in identifying ARMH2 also provides valuable approaches for discovering uncharacterized components in other protein complexes.
- Fudan University, Shanghai, China.
Organizational Affiliation: