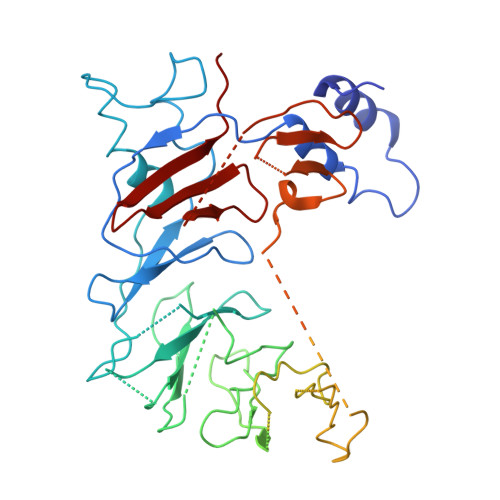
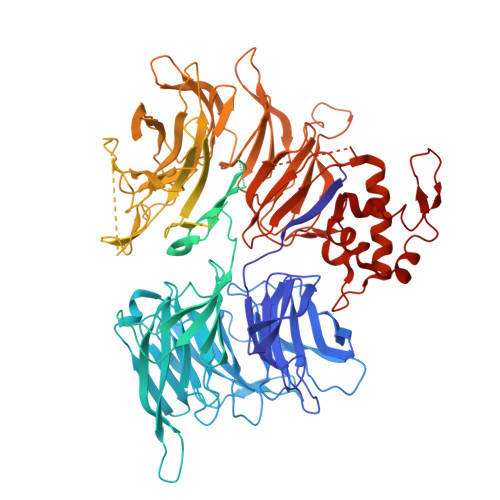
Cryo-EM structure of the human COP1-DET1 ubiquitin ligase complex.
Wang, S., Teng, F., Stjepanovic, G., Rao, F., Su, M.Y.(2026) Nat Commun 17: 543-543
- PubMed: 41540009
- DOI: https://doi.org/10.1038/s41467-026-68375-7
- Primary Citation of Related Structures:
9LTJ, 9LTL, 9LTO, 9LTR, 9LTW, 9LTZ, 9LU1, 9LUL, 9M0Y, 9W90 - PubMed Abstract:
Ubiquitin modifications regulate fundamental cellular activities by modulating protein stability and function. The ubiquitin ligase COP1, which is present across species from plants to humans, plays a crucial role in the ubiquitination of developmental transcription factors. While COP1 can function independently, it can also be incorporated into CULLIN4-RING ubiquitin ligase (CRL4) complexes through the DET1 adaptor protein. Despite its biological significance, the structural and functional mechanisms of COP1 and DET1-containing complexes remains poorly understood. Here we present the cryo-electron microscopy structures of human COP1 in complex with DDB1-DDA1-DET1 and Ube2e2, revealing an inactive stacked assembly state. Co-expression with COP1 substrates including c-Jun or ETS2 disrupts this configuration, inducing a conformational rearrangement into a distinct dimeric state that allows substrate access. Structural modelling identifies the spatial organization of COP1 WD40 domains where substrate recruits. DET1 serves as a structural scaffold, bridging COP1 and Ube2e2 to initiate potential ubiquitin addition on substrates, while DDB1 recruits the CULLIN4-RBX1 complex to facilitate Ube2d3-mediated ubiquitin chain elongation. These results reveal the dynamic interplay between the structural states of the CRL4 DET1-COP1 E3 ligase complex and its substrate specific activation mechanism, offering mechanistic insights into ubiquitination regulation and a basis for future studies on E3 ligase dynamics.
- Department of Biochemistry, SUSTech Homeostatic Medicine Institute, School of Medicine, Southern University of Science and Technology, Shenzhen, China.
Organizational Affiliation: