Structural studies on the conformation changes induced by ligand binding in an Adenine phosphoribosyltransferase (FnAPRT) from Fusobacterium nucleatum
Kim, B., Hwang, J., Do, H., Lee, J.H.To be published.
Experimental Data Snapshot
Starting Model: in silico
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Adenine phosphoribosyltransferase | A [auth B] | 170 | Fusobacterium nucleatum | Mutation(s): 0 Gene Names: apt, FN1483 EC: 2.4.2.7 | 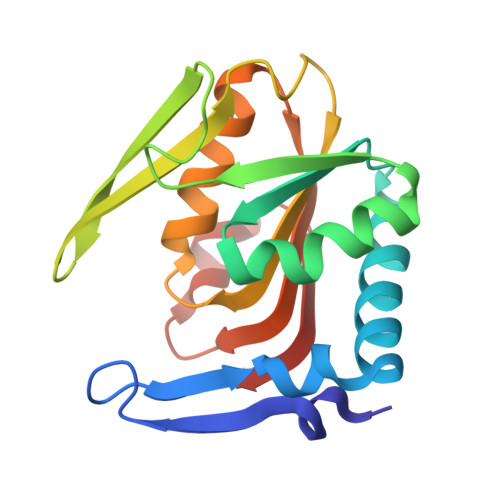 |
UniProt | |||||
Find proteins for Q8RDM9 (Fusobacterium nucleatum subsp. nucleatum (strain ATCC 25586 / DSM 15643 / BCRC 10681 / CIP 101130 / JCM 8532 / KCTC 2640 / LMG 13131 / VPI 4355)) Explore Q8RDM9 Go to UniProtKB: Q8RDM9 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8RDM9 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| AMP (Subject of Investigation/LOI) Query on AMP | C [auth B] | ADENOSINE MONOPHOSPHATE C10 H14 N5 O7 P UDMBCSSLTHHNCD-KQYNXXCUSA-N |  | ||
| PO4 (Subject of Investigation/LOI) Query on PO4 | B | PHOSPHATE ION O4 P NBIIXXVUZAFLBC-UHFFFAOYSA-K |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 73.048 | α = 90 |
| b = 106.523 | β = 90 |
| c = 47.807 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| PHENIX | refinement |
| XDS | data reduction |
| XDS | data scaling |
| MOLREP | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Research Foundation (NRF, Korea) | Korea, Republic Of | -- |