Complex formation via regulatory component of MMOH from Methylosinus sporium 5
Hwang, Y., Pozharski, E., Lee, S.J.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Methane monooxygenase | 526 | Methylosinus sporium | Mutation(s): 0 | 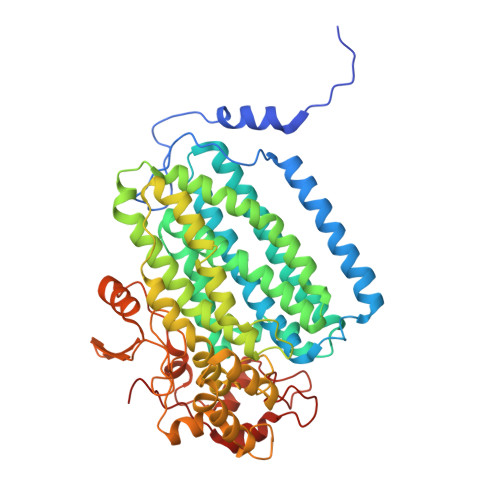 | |
UniProt | |||||
Find proteins for Q27RN7 (Methylosinus sporium) Explore Q27RN7 Go to UniProtKB: Q27RN7 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q27RN7 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Methane monooxygenase | 395 | Methylosinus sporium | Mutation(s): 0 | 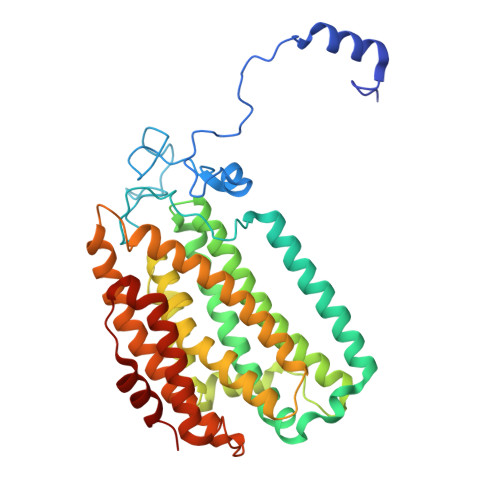 | |
UniProt | |||||
Find proteins for Q27RN6 (Methylosinus sporium) Explore Q27RN6 Go to UniProtKB: Q27RN6 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q27RN6 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Methane monooxygenase | 169 | Methylosinus sporium | Mutation(s): 0 | 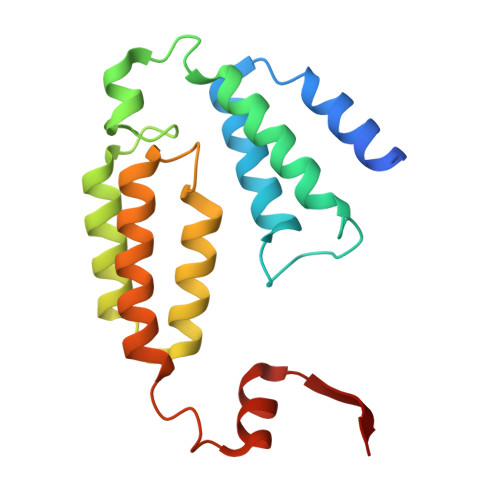 | |
UniProt | |||||
Find proteins for Q27RN4 (Methylosinus sporium) Explore Q27RN4 Go to UniProtKB: Q27RN4 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q27RN4 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| FE (Subject of Investigation/LOI) Query on FE | G [auth A], H [auth A], I [auth D], J [auth D] | FE (III) ION Fe VTLYFUHAOXGGBS-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.19.2-4158 |
| RECONSTRUCTION | cryoSPARC | 3.3.2 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Research Foundation (NRF, Korea) | Korea, Republic Of | 2023RIS-008 |
| Ministry of Education (MoE, Korea) | Korea, Republic Of | 2017R1A6A1A03015876 |