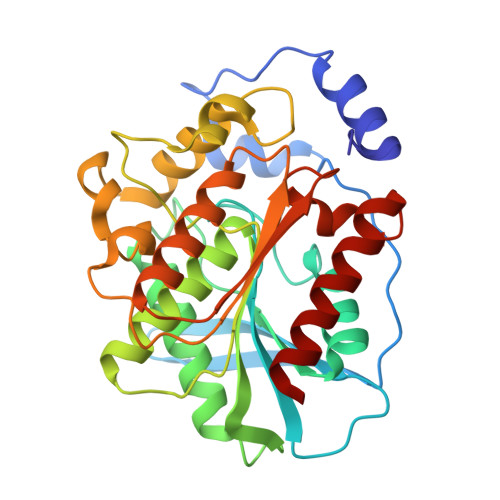
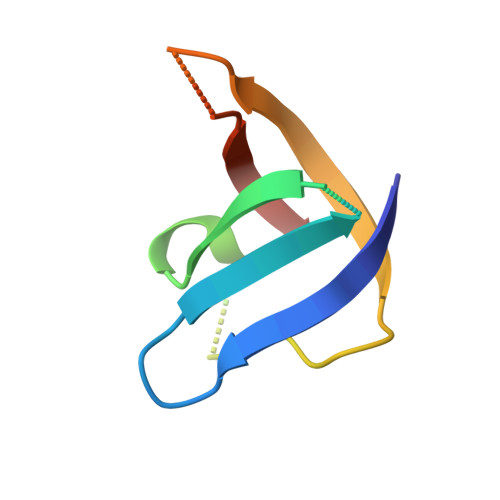
Heat-sterilizable antibody mimics designed on the cold shock protein scaffold from hyperthermophile Thermotoga maritima.
Amesaka, H., Tachibana, M., Hara, M., Toya, S., Nakagawa, H., Matsumura, H., Hirata, A., Fujihashi, M., Takano, K., Tanaka, S.I.(2025) Protein Sci 34: e70018-e70018
- PubMed: 39724358
- DOI: https://doi.org/10.1002/pro.70018
- Primary Citation of Related Structures:
9JU4 - PubMed Abstract:
Antibodies and antibody mimics are extensively used in the pharmaceutical industry, where stringent safety standards are required. Implementing heat sterilization during or after the manufacturing process could help prevent contamination by viruses and bacteria. However, conventional antibodies and antibody mimics are not suitable for heat sterilization because they irreversibly denature at high temperatures. In this study, we focused on the refolding property of the cold shock protein from the hyperthermophile Thermotoga maritima (TmCSP), which denatures at elevated temperatures but regains its native structure upon re-cooling. We designed and constructed a mutant library of TmCSP in which amino acid residues in its three surface loops were diversified. From the library, mutant TmCSPs that bind to each of eight target proteins were selected by phage and yeast surface display methods. We confirmed that the secondary structure and binding affinity of all the selected mutants were restored after heat treatment followed by cooling. Additionally, freeze-drying did not impair their binding affinity. The crystal structure of a mutant TmCSP in complex with its target, the esterase from Alicyclobacillus acidocaldarius, revealed specific interactions between them. These results clearly demonstrate the feasibility of creating heat-sterilizable antibody mimics using TmCSP as a scaffold.
- Graduate School of Life and Environmental Sciences, Kyoto Prefectural University, Kyoto, Japan.
Organizational Affiliation: