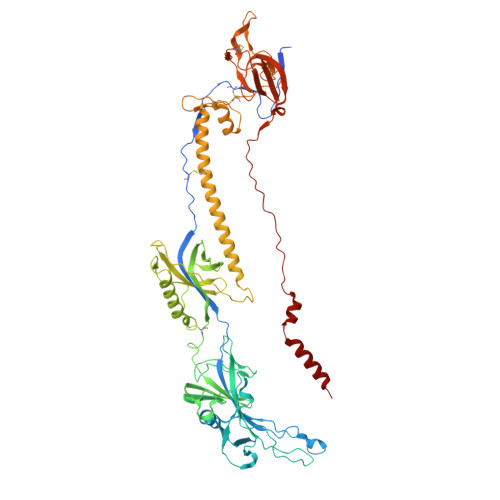
Human herpesvirus 6B glycoprotein B postfusion structure, vulnerability mapping, and receptor recognition.
Xie, C., Fang, X.Y., Liu, Y.T., Tian, X.S., Zhong, L.Y., Wu, P.H., Zhou, H., Li, P.L., Yang, Y.L., Jiang, Z.Y., Sui, S.F., Liu, Z., Zeng, M.S., Sun, C.(2025) PLoS Pathog 21: e1013300-e1013300
- PubMed: 40632757
- DOI: https://doi.org/10.1371/journal.ppat.1013300
- Primary Citation of Related Structures:
9JLI - PubMed Abstract:
Human herpesvirus 6B (HHV-6B), a β-herpesvirus that significantly threatens immunocompromised individuals, currently lacks targeted antiviral therapies or vaccines. Glycoprotein B (gB), the primary mediator of membrane fusion during viral entry, is a key target for neutralizing antibody (nAb) and vaccine development. In this study, we determined a 2.8 Å cryo-EM structure of the HHV-6B gB ectodomain in its postfusion conformation, unveiling unique N-terminal features and resolving the furin site for the first time in herpesviruses. Comparative analyses highlighted similarities between HHV-6B gB and gB from human cytomegalovirus (HCMV) and Epstein-Barr virus (EBV), mapping conserved residues across herpesviruses. Cross-binding assays indicated minimal cross-epitope recognition by nAbs from other herpesviruses, while several potential vulnerable sites on HHV-6B gB were identified. These insights advance our understanding of HHV-6B infection mechanisms and support future development of antibodies or vaccines targeting gB.
- State Key Laboratory of Oncology in South China, Guangdong Provincial Clinical Research Center for Cancer, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Sun Yat-sen University Cancer Center, Guangzhou, China.
Organizational Affiliation: