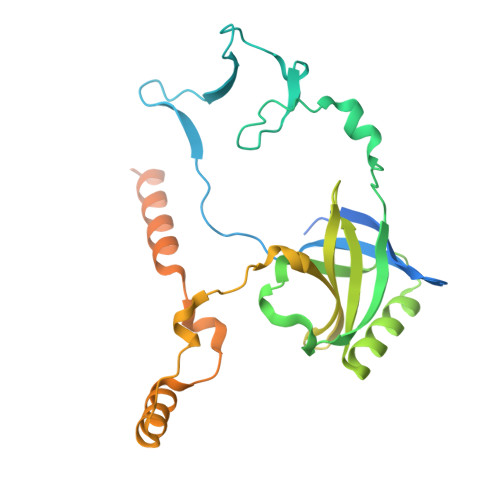
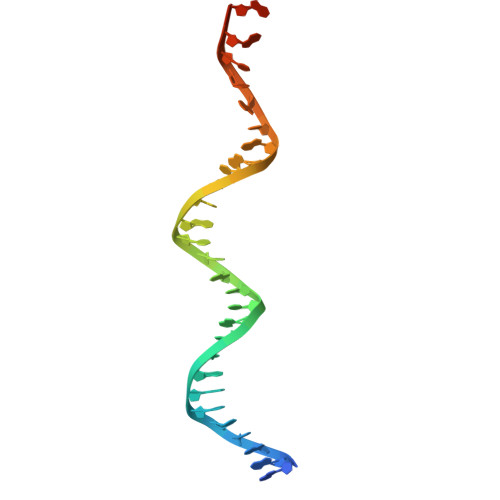
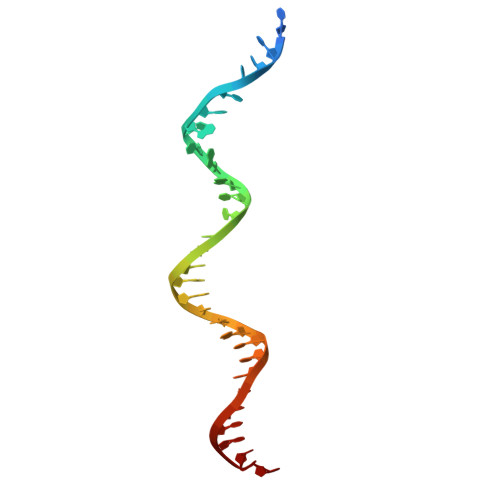
Oligomerisation of Ku from Mycobacterium tuberculosis promotes DNA synapsis.
Zahid, S., Baconnais, S., Smith, H., Atwal, S., Bates, L., Read, H., Chadda, A., Morati, F., Bedwell, T., Stender, E.G.P., Walter, J., Hardwick, S.W., Westerlund, F., Galburt, E., Le Cam, E., Pyne, A., Mukamolova, G.V., Chaplin, A.K.(2025) Nat Commun 16: 10568-10568
- PubMed: 41298423
- DOI: https://doi.org/10.1038/s41467-025-65609-y
- Primary Citation of Related Structures:
9I91 - PubMed Abstract:
Mycobacterium tuberculosis (Mtb), the causative agent of tuberculosis (TB), is estimated to infect nearly one-quarter of the global population. A key factor in its resilience and persistence is its robust DNA repair capacity. Non-homologous end joining (NHEJ) is the primary pathway for repairing DNA double-strand breaks (DSBs) in many organisms, including Mtb, where it is mediated by the Ku protein and the multifunctional LigD enzyme. In this study, we demonstrate that Ku is essential for mycobacterial survival under DNA-damaging conditions. Using cryogenic electron microscopy (cryo-EM), we solved high-resolution structures of both the apo and DNA-bound forms of the Ku-Mtb homodimer. Our structural and biophysical analyses reveal that Ku forms an extended proteo-filament upon binding DNA. We identify critical residues involved in filament formation and DNA synapsis and show that their mutation severely impairs bacterial viability. Furthermore, we propose a model in which the C-terminus of Ku regulates DNA binding and loading and facilitates subsequent recruitment of LigD. These findings provide unique insights into bacterial DNA repair and guide future therapeutics.
- Leicester Institute for Structural and Chemical Biology, Department of Molecular and Cell Biology, University of Leicester, Leicester, UK.
Organizational Affiliation: