Structural insights into cohesin cleavage by human separase
Yu, J., Schmidt, S., Botto, M., Ghent, C.M., Morgan, D.O., Boland, A.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Double-strand-break repair protein rad21 homolog | A [auth B] | 227 | Homo sapiens | Mutation(s): 0 Gene Names: RAD21, HR21, KIAA0078, NXP1, SCC1 |  |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O60216 (Homo sapiens) Explore O60216 Go to UniProtKB: O60216 | |||||
PHAROS: O60216 GTEx: ENSG00000164754 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O60216 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Separin | B [auth A] | 2,172 | Homo sapiens | Mutation(s): 1 Gene Names: ESPL1, ESP1, KIAA0165 EC: 3.4.22.49 | 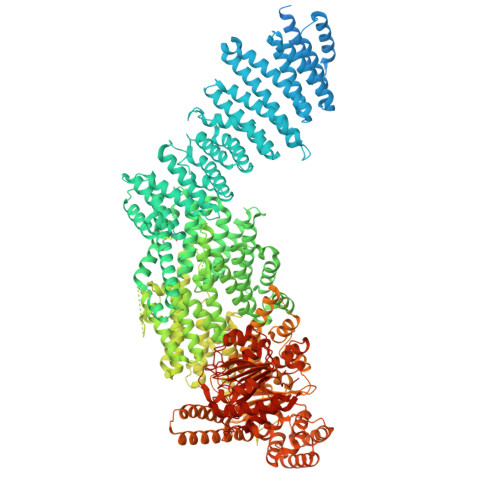 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q14674 (Homo sapiens) Explore Q14674 Go to UniProtKB: Q14674 | |||||
PHAROS: Q14674 GTEx: ENSG00000135476 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q14674 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ZN (Subject of Investigation/LOI) Query on ZN | C [auth A] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| SEP Query on SEP | A [auth B] | L-PEPTIDE LINKING | C3 H8 N O6 P |  | SER |
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.20.1_4487 |
| MODEL REFINEMENT | Coot | 0.9.8.92 |
| RECONSTRUCTION | cryoSPARC | 4.4.0 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Swiss National Science Foundation | Switzerland | 310030_185235 |
| Swiss National Science Foundation | Switzerland | TMSGI3_211581 |