NMR solution structure of RPRD2 CTD-interacting domain and pS2,7 RNAPII CTD peptide.
Linhartova, K., Macosek, J., Kubicek, K., Smirakova, E., Steel, R.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Regulation of nuclear pre-mRNA domain-containing protein 2 | 148 | Homo sapiens | Mutation(s): 0 Gene Names: RPRD2, KIAA0460, HSPC099 | 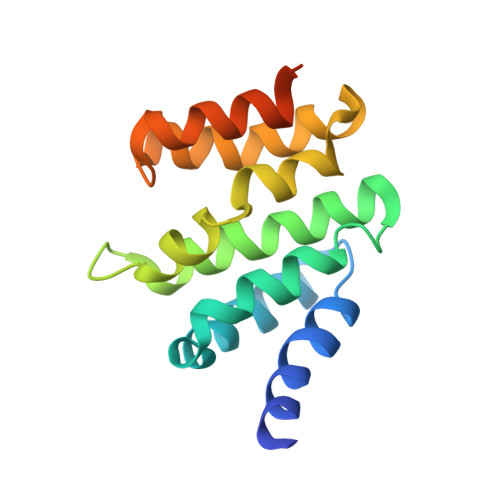 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q5VT52 (Homo sapiens) Explore Q5VT52 Go to UniProtKB: Q5VT52 | |||||
PHAROS: Q5VT52 GTEx: ENSG00000163125 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q5VT52 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| DNA-directed RNA polymerase II subunit RPB1 | 11 | Homo sapiens | Mutation(s): 0 EC: 2.7.7.6 (PDB Primary Data), 3.1.13 (PDB Primary Data), 2.7.7.48 (PDB Primary Data) |  | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P24928 (Homo sapiens) Explore P24928 Go to UniProtKB: P24928 | |||||
PHAROS: P24928 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P24928 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Modified Residues 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Type | Formula | 2D Diagram | Parent |
| SEP Query on SEP | B | L-PEPTIDE LINKING | C3 H8 N O6 P |  | SER |
| Funding Organization | Location | Grant Number |
|---|---|---|
| European Research Council (ERC) | European Union | 649030 |
| Czech Science Foundation | Czech Republic | GA22-19896S |
| Ministry of Education (MoE, Czech Republic) | Czech Republic | CZ.02.01.01/00/22_008/0004575 |