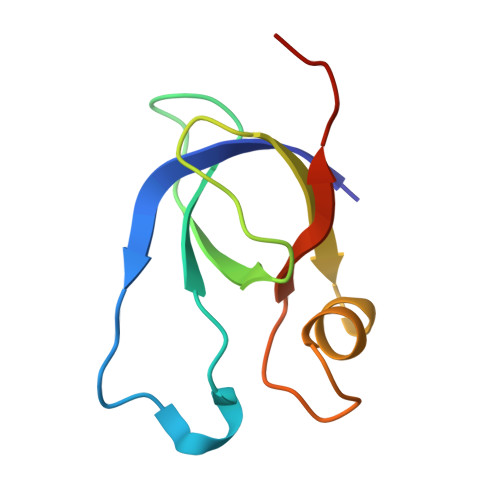
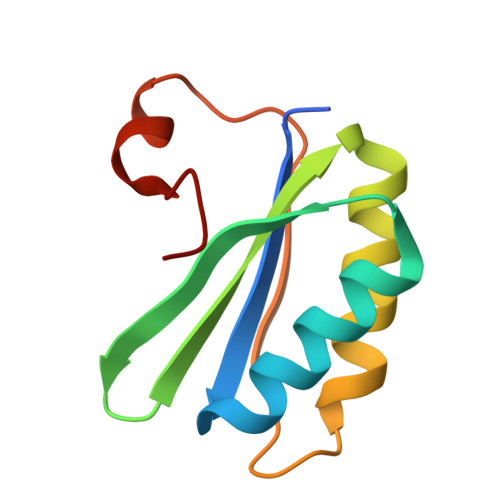
Structure and encapsulation of carbonic anhydrase within the alpha-carboxysome.
Ng, P.C., Adegbite, O., Li, T., Basle, A., Marles-Wright, J., Liu, L.N.(2025) Proc Natl Acad Sci U S A 122: e2523723122-e2523723122
- PubMed: 41223214
- DOI: https://doi.org/10.1073/pnas.2523723122
- Primary Citation of Related Structures:
9G4T, 9GVC, 9GW1 - PubMed Abstract:
Carboxysomes in cyanobacteria and certain proteobacteria enable efficient CO 2 fixation by encapsulating ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) and carbonic anhydrase (CA) within a semipermeable shell. Sequestered CA catalyze the rapid interconversion of CO 2 and HCO 3 - , supplying elevated levels of CO 2 to boost Rubisco carboxylation. Despite its essential role, the structure and encapsulation of CA within carboxysomes remain poorly understood. Here, we determined the molecular structure of α-carboxysomal CA from the model chemoautotrophic bacterium Halothiobacillus neapolitanus ( Hn CsoSCA). Hn CsoSCA adopts a trimer-of-dimers oligomeric structure without the incorporation of a zinc ion at its symmetric center. Using synthetic minishells, we demonstrate that Hn CsoSCA interacts with the CsoS1A shell hexamer and is incorporated into the minishells at the inner surface, independent of the CsoS2 linker protein. Hn CsoSCA truncations suggest nonspecific interactions between Hn CsoSCA and CsoS1A. We further show that Hn CsoSCA bridges Rubisco and the shell facets. Our study offers insights into the assembly and encapsulation mechanisms of α-carboxysomes and provides the framework for reprogramming carboxysome structures for synthetic biology and biotechnological applications.
- Institute of Systems, Molecular and Integrative Biology, University of Liverpool, Liverpool L69 7ZB, United Kingdom.
Organizational Affiliation: