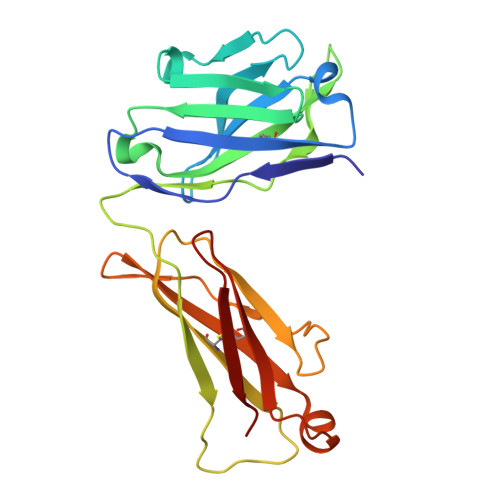
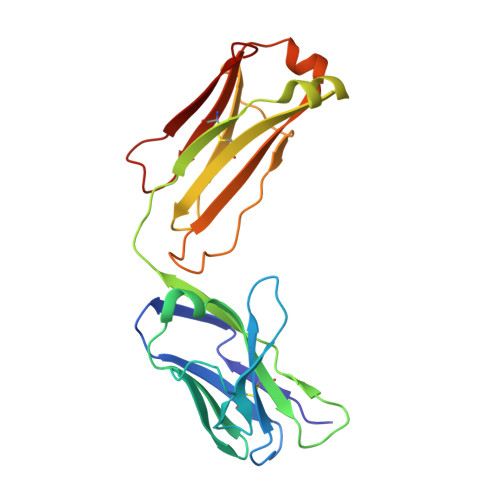
Defective cell-autonomous signalling and antigenic polyreactivity of B-cell receptors from chronic lymphocytic leukaemia stereotyped subset 1.
Cocomazzi, P.G., Iatrou, A., Minici, C., Gounari, M., Linder, A.T., Akpinaroglu, C., Patrone, M., Broggini, L., Frenquelli, M., Sarrigeorgiou, I., Lymberi, P., Petrakis, G., Koletsa, T., Scarfo, L., Agathangelidis, A., Jumaa, H., Maity, P.C., Ghia, P., Stamatopoulos, K., Degano, M.(2025) Nat Commun
- PubMed: 41469389
- DOI: https://doi.org/10.1038/s41467-025-68040-5
- Primary Citation of Related Structures:
9GFF, 9GFG, 9GFH - PubMed Abstract:
A shared feature of B-cell receptors (BCRs) expressed on neoplastic B cells from patients with chronic lymphocytic leukaemia (CLL) concerns their continual activation of intracellular signalling without requiring external antigens. This autonomous signalling mechanism has previously been demonstrated to arise from BCR-BCR homotypic interactions in three distinct stereotyped CLL subsets (2, 4 and 169). CLL subset 1 is the second largest stereotyped subset, epitomizing unmutated CLL and known for a particularly aggressive clinical course. Here we show that, despite their significant sequence similarity, BCRs originating from three different subset 1 CLL cases exhibit variations in their combining site structures and associated physicochemical properties. Subset 1 BCRs are characterized by a common reactivity pattern towards various autoantigens, though they maintain distinct receptor-specific characteristics. Analysis of crystal structures of the respective BCR Fab fragments reveals lack of conservation in intermolecular crystal contacts, paralleled by no self-association in solution and incapacity to induce intracellular signalling when expressed in a model B-cell line. These findings suggest that cell-autonomous signalling may not be universally present across all CLL, implying the existence of other mechanisms in sustaining leukemic cell proliferation and CLL progression mediated by BCR signalling.
- Biocrystallography Unit, Department of Immunology, Transplantation and Infectious Diseases, IRCCS Ospedale San Raffaele, 20132, Milano, Italy.
Organizational Affiliation: