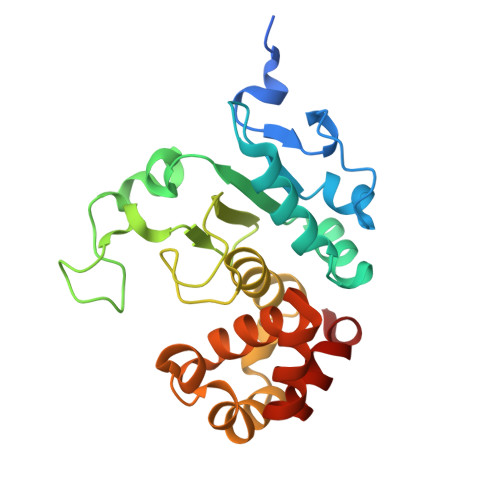
Structural analyses and substrate profiling of PPEP-3 provide new insights into the molecular basis of Pro-Pro endopeptidase specificity
Claushuis, B., Wojtalla, F., Papenhagen, L., Cordfunke, R.A., de Ru, A.H., van Leeuwen, H.C., Corver, J., Hensbergen, P.J., Baumann, U.(2026) iScience 29: 114360