Room temperature structure of Aspartyl/Asparaginyl beta-hydroxylase (AspH) in complex with Fe, 2-oxoglutarate and Factor X derived peptide fragment
Brasnett, A., de Munnik, M., Brewitz, L., Rabe, P., Schofield, C.J., Kern, J.F.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Aspartyl/asparaginyl beta-hydroxylase | 428 | Homo sapiens | Mutation(s): 0 Gene Names: ASPH, BAH EC: 1.14.11.16 | 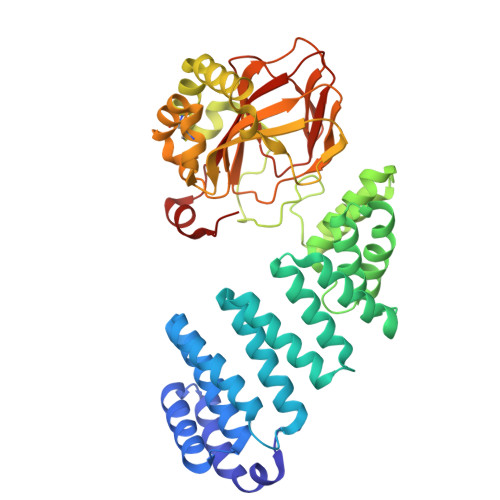 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q12797 (Homo sapiens) Explore Q12797 Go to UniProtKB: Q12797 | |||||
PHAROS: Q12797 GTEx: ENSG00000198363 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q12797 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Coagulation factor X | 39 | Homo sapiens | Mutation(s): 4 EC: 3.4.21.6 | 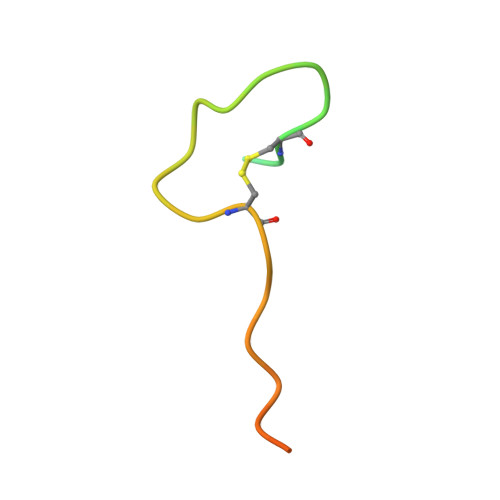 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P00742 (Homo sapiens) Explore P00742 Go to UniProtKB: P00742 | |||||
PHAROS: P00742 GTEx: ENSG00000126218 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P00742 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| AKG (Subject of Investigation/LOI) Query on AKG | C [auth A] | 2-OXOGLUTARIC ACID C5 H6 O5 KPGXRSRHYNQIFN-UHFFFAOYSA-N |  | ||
| FE (Subject of Investigation/LOI) Query on FE | D [auth A] | FE (III) ION Fe VTLYFUHAOXGGBS-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 50.796 | α = 90 |
| b = 88.16 | β = 90 |
| c = 125.161 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| cctbx.xfel | data reduction |
| cctbx.xfel.merge | data scaling |
| PHASER | phasing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Biotechnology and Biological Sciences Research Council (BBSRC) | United Kingdom | BB/V003291/1 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | 1R35GM149528-01 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM110501 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM126289 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | GM117126 |