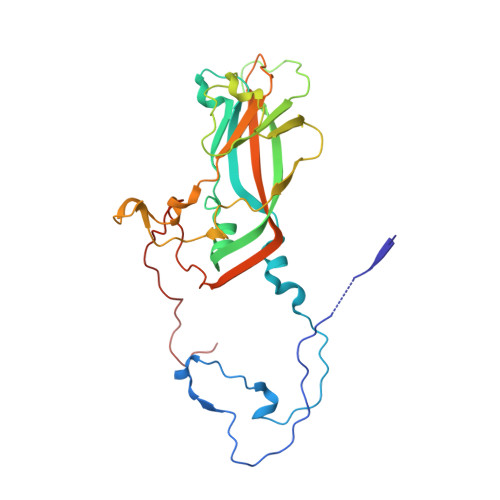
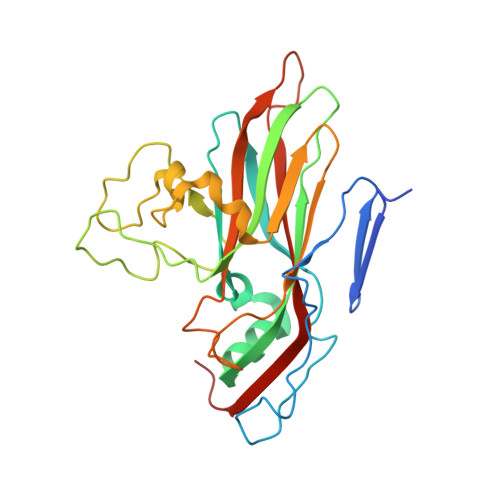
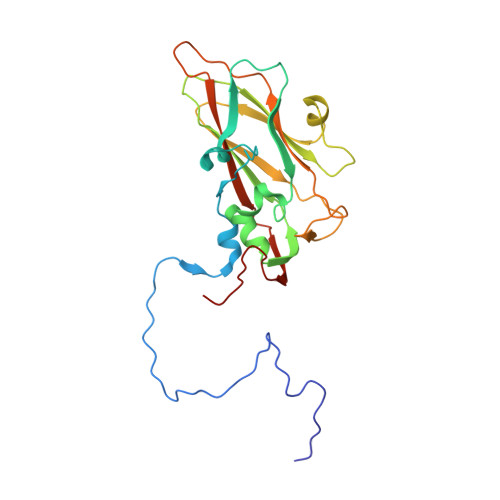
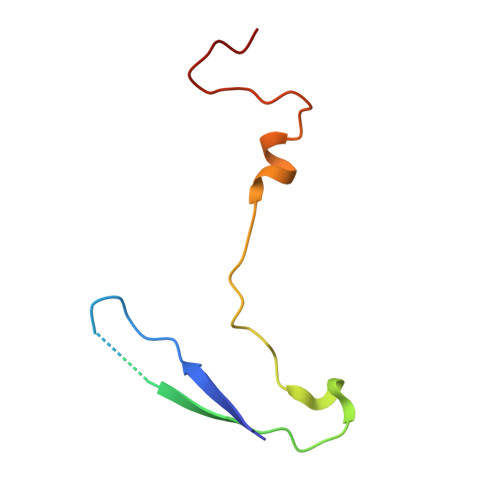
SAR Analysis of Novel Coxsackie virus A9 Capsid Binders.
Tammaro, C., Plavec, Z., Myllymaki, L., Mitchell, C., Consalvi, S., Biava, M., Ciogli, A., Domanska, A., Leppilampi, V., Buckner, C., Manetto, S., Scio, P., Coluccia, A., Laajala, M., Dondio, G.M., Bigogno, C., Marjomaki, V., Butcher, S.J., Poce, G.(2024) J Med Chem 67: 17144-17161
- PubMed: 39292620
- DOI: https://doi.org/10.1021/acs.jmedchem.4c00701
- Primary Citation of Related Structures:
8S7J, 9EXI, 9FA9, 9FCZ, 9FGN, 9FO2, 9FO5, 9FP5 - PubMed Abstract:
Enterovirus infections are common in humans, yet there are no approved antiviral treatments. In this study we concentrated on inhibition of one of the Enterovirus B (EV-B), namely Coxsackievirus A9 (CVA9), using a combination of medicinal chemistry, virus inhibition assays, structure determination from cryogenic electron microscopy and molecular modeling, to determine the structure activity relationships for a promising class of novel N -phenylbenzylamines. Of the new 29 compounds synthesized, 10 had half maximal effective concentration (EC 50 ) values between 0.64-10.46 μM, and of these, 7 had 50% cytotoxicity concentration (CC 50 ) values higher than 200 μM. In addition, this new series of compounds showed promising physicochemical properties and act through capsid stabilization, preventing capsid expansion and subsequent release of the genome.
- Department of Drug Chemistry and Technologies, Sapienza University of Rome, 00185 Rome, Italy.
Organizational Affiliation: