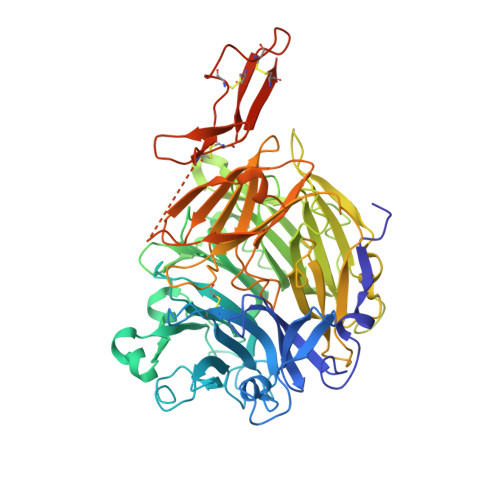
CRTAC1 has a Compact beta-propeller-TTR Core Stabilized by Potassium Ions.
Beugelink, J.W., Hof, H., Janssen, B.J.C.(2024) J Mol Biol 436: 168712-168712
- PubMed: 39029889
- DOI: https://doi.org/10.1016/j.jmb.2024.168712
- Primary Citation of Related Structures:
9ETN - PubMed Abstract:
Cartilage acidic protein-1 (CRTAC1) is a secreted glycoprotein with roles in development, function and repair of the nervous system. It is linked to ischemic stroke, osteoarthritis and (long) COVID outcomes, and has suppressive activity in carcinoma and bladder cancer. Structural characterization of CRTAC1 has been complicated by its tendency to form disulfide-linked aggregates. Here, we show that CRTAC1 is stabilized by potassium ions. Using x-ray crystallography, we determined the structure of CRTAC1 to 1.6 Å. This reveals that the protein consists of a three-domain fold, including a previously-unreported compact β-propeller-TTR combination, in which an extended loop of the TTR plugs the β-propeller core. Electron density is observed for ten bound ions: six calcium, three potassium and one sodium. Low potassium ion concentrations lead to changes in tryptophan environment and exposure of two buried free cysteines located on a β-blade and in the β-propeller-plugging TTR loop. Mutating the two free cysteines to serines prevents covalent intermolecular interactions, but not aggregation, in absence of potassium ions. The potassium ion binding sites are located between the blades of the β-propeller, explaining their importance for the stability of the CRTAC1 fold. Despite varying in sequence, the three potassium ion binding sites are structurally similar and conserved features of the CRTAC protein family. These insights into the stability and structure of CRTAC1 provide a basis for further work into the function of CRTAC1 in health and disease.
Organizational Affiliation:
Structural Biochemistry, Bijvoet Centre for Biomolecular Research, Faculty of Science, Utrecht University, Universiteitsweg 99, 3584 CG Utrecht, the Netherlands.