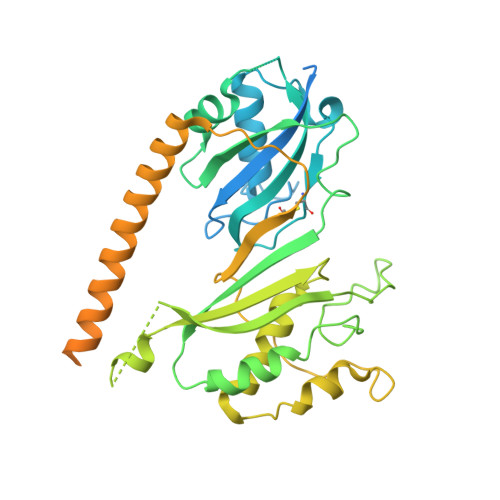
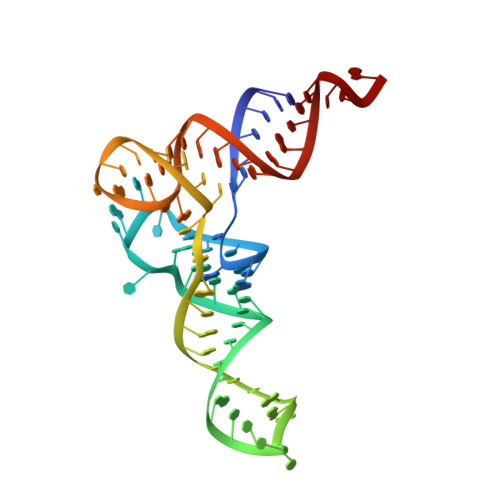
The molecular basis of tRNA selectivity by human pseudouridine synthase 3.
Lin, T.Y., Kleemann, L., Jezowski, J., Dobosz, D., Rawski, M., Indyka, P., Wazny, G., Mehta, R., Chramiec-Glabik, A., Koziej, L., Ranff, T., Fufezan, C., Wawro, M., Kochan, J., Bereta, J., Leidel, S.A., Glatt, S.(2024) Mol Cell 84: 2472-2489.e8
- PubMed: 38996458
- DOI: https://doi.org/10.1016/j.molcel.2024.06.013
- Primary Citation of Related Structures:
8OKD, 9ENB, 9ENC, 9ENE, 9ENF, 9F9Q - PubMed Abstract:
Pseudouridine (Ψ), the isomer of uridine, is ubiquitously found in RNA, including tRNA, rRNA, and mRNA. Human pseudouridine synthase 3 (PUS3) catalyzes pseudouridylation of position 38/39 in tRNAs. However, the molecular mechanisms by which it recognizes its RNA targets and achieves site specificity remain elusive. Here, we determine single-particle cryo-EM structures of PUS3 in its apo form and bound to three tRNAs, showing how the symmetric PUS3 homodimer recognizes tRNAs and positions the target uridine next to its active site. Structure-guided and patient-derived mutations validate our structural findings in complementary biochemical assays. Furthermore, we deleted PUS1 and PUS3 in HEK293 cells and mapped transcriptome-wide Ψ sites by Pseudo-seq. Although PUS1-dependent sites were detectable in tRNA and mRNA, we found no evidence that human PUS3 modifies mRNAs. Our work provides the molecular basis for PUS3-mediated tRNA modification in humans and explains how its tRNA modification activity is linked to intellectual disabilities.
- Małopolska Centre of Biotechnology, Jagiellonian University, 30-387 Kraków, Poland. Electronic address: ting-yu.lin@durham.ac.uk.
Organizational Affiliation: