Mechanism of allosteric inhibition of RUVBL1-RUVBL2 by the small-molecule CB-6644
Garcia-Martin, C., Lopez-Perrote, A., Boskovic, J., Llorca, O.(2024) Cell Rep Phys Sci
Experimental Data Snapshot
Starting Model: in silico
View more details
wwPDB Validation 3D Report Full Report
(2024) Cell Rep Phys Sci
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| RuvB-like 1 | A, C [auth B], D [auth C] | 459 | Homo sapiens | Mutation(s): 0 Gene Names: RUVBL1, INO80H, NMP238, TIP49, TIP49A EC: 3.6.4.12 | 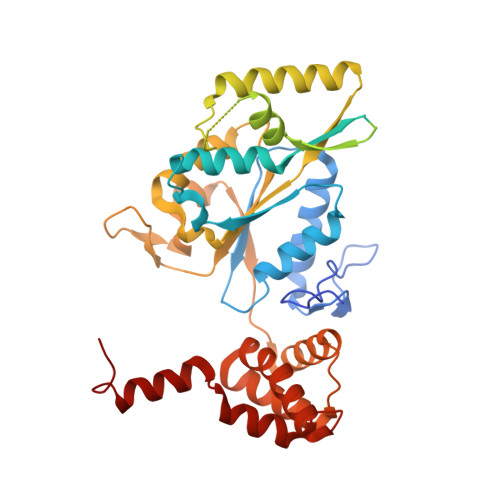 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9Y265 (Homo sapiens) Explore Q9Y265 Go to UniProtKB: Q9Y265 | |||||
PHAROS: Q9Y265 GTEx: ENSG00000175792 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9Y265 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| RuvB-like 2 | B [auth D], E [auth F], F [auth E] | 481 | Homo sapiens | Mutation(s): 0 Gene Names: RUVBL2, INO80J, TIP48, TIP49B, CGI-46 EC: 3.6.4.12 | 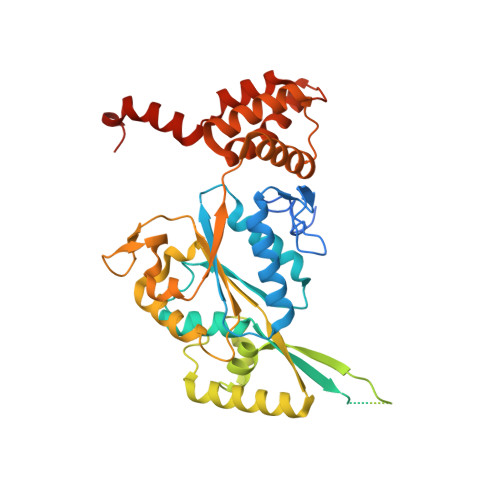 |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q9Y230 (Homo sapiens) Explore Q9Y230 Go to UniProtKB: Q9Y230 | |||||
PHAROS: Q9Y230 GTEx: ENSG00000183207 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q9Y230 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| ATP (Subject of Investigation/LOI) Query on ATP | G [auth A] I [auth D] K [auth B] M [auth C] O [auth F] | ADENOSINE-5'-TRIPHOSPHATE C10 H16 N5 O13 P3 ZKHQWZAMYRWXGA-KQYNXXCUSA-N |  | ||
| MG (Subject of Investigation/LOI) Query on MG | H [auth A] J [auth D] L [auth B] N [auth C] P [auth F] | MAGNESIUM ION Mg JLVVSXFLKOJNIY-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | ISOLDE | 1.7 |
| MODEL REFINEMENT | PHENIX | 1.20.1-4487 |
| MODEL REFINEMENT | Coot | 0.9.8.5 |
| RECONSTRUCTION | RELION | 4.0 |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Agencia Estatal de Investigacion (AEI) | Spain | PID2020-114429RB-I00 |