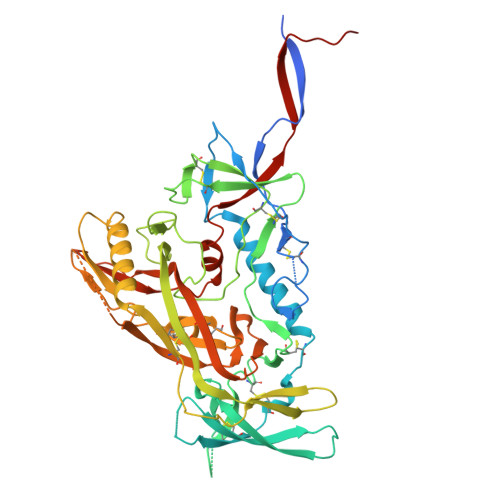
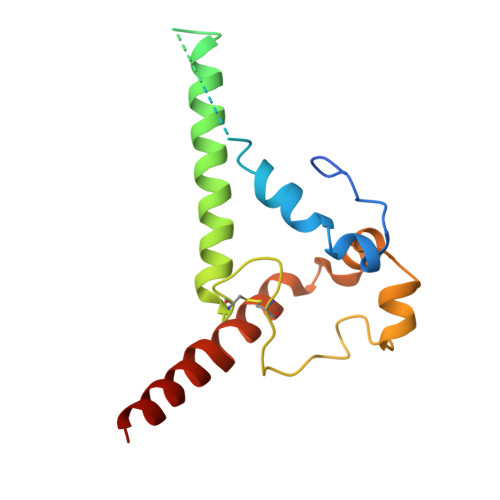
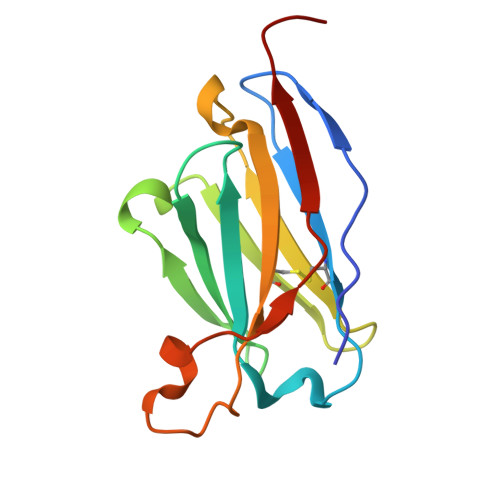
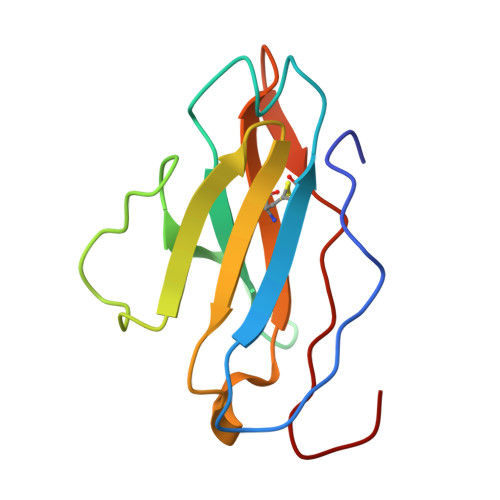
Mapping essential somatic hypermutations in a CD4-binding site bNAb informs HIV-1 vaccine design.
Dam, K.A., Gristick, H.B., Li, Y.E., Yang, Z., Gnanapragasam, P.N.P., West Jr., A.P., Seaman, M.S., Bjorkman, P.J.(2025) Cell Rep 44: 115713-115713
- PubMed: 40378041
- DOI: https://doi.org/10.1016/j.celrep.2025.115713
- Primary Citation of Related Structures:
9EHL, 9EHM - PubMed Abstract:
HIV-1 broadly neutralizing antibodies (bNAbs) targeting the CD4-binding site (CD4bs) contain rare features that pose challenges to elicit these bNAbs through vaccination. The IOMA class of CD4bs bNAbs includes fewer rare features and somatic hypermutations (SHMs) to achieve broad neutralization, thus presenting a potentially accessible pathway for vaccine-induced bNAb development. Here, we created a library of IOMA variants in which each SHM was individually reverted to the inferred germline counterpart to investigate the roles of SHMs in conferring IOMA's neutralization potency and breadth. Impacts on neutralization for each variant were evaluated, and this information was used to design minimally mutated IOMA-class variants (IOMAmin) that incorporated the fewest SHMs required for achieving IOMA's neutralization breadth. A cryoelectron microscopy (cryo-EM) structure of an IOMAmin variant bound to Env was used to further interpret characteristics of IOMA variants to elucidate how IOMA's structural features correlate with its neutralization mechanism, informing the design of IOMA-targeting immunogens.
- Division of Biology and Biological Engineering, California Institute of Technology, Pasadena, CA 91125, USA.
Organizational Affiliation: