Structural Insights into De Novo Promoter Escape by Mycobacterium tuberculosis RNA Polymerase.
Brewer, J., Delbeau, M., Zoullas, W.B., Darst, S.A., Campbell, E.A.(2025) Nat Commun 16: 9990-9990
- PubMed: 41233305
- DOI: https://doi.org/10.1038/s41467-025-64941-7
- Primary Citation of Related Structures:
9E7V, 9E7Y, 9E84, 9E85, 9E86, 9E87, 9E88 - PubMed Abstract:
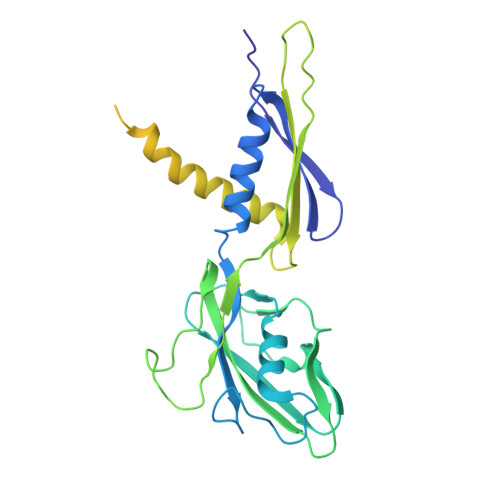
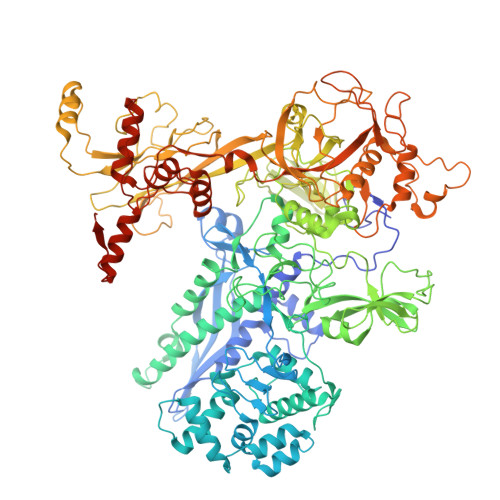
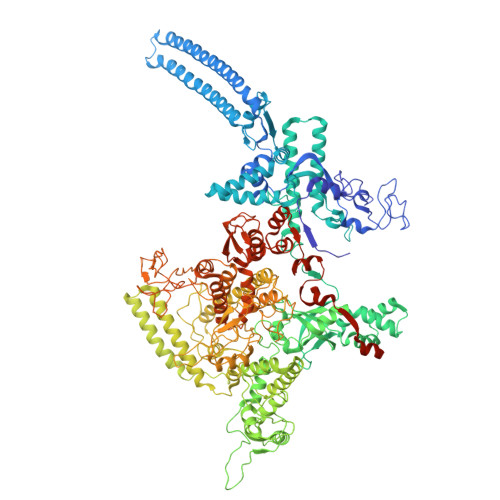
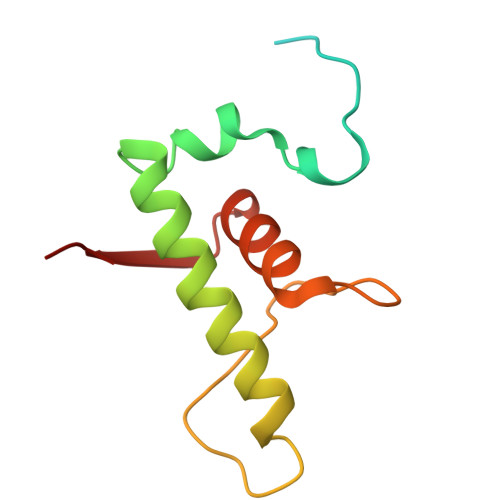
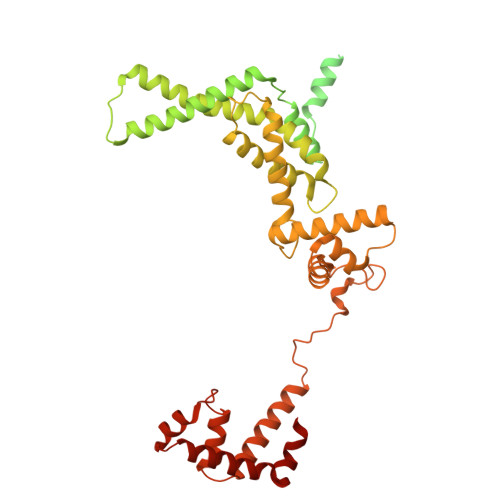
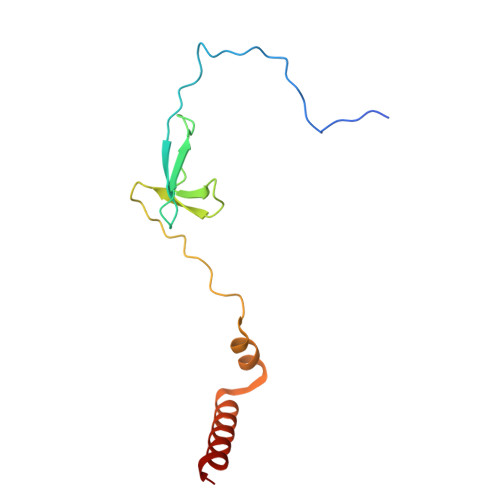
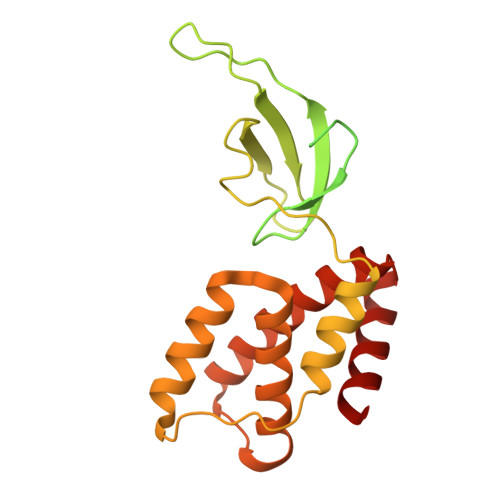
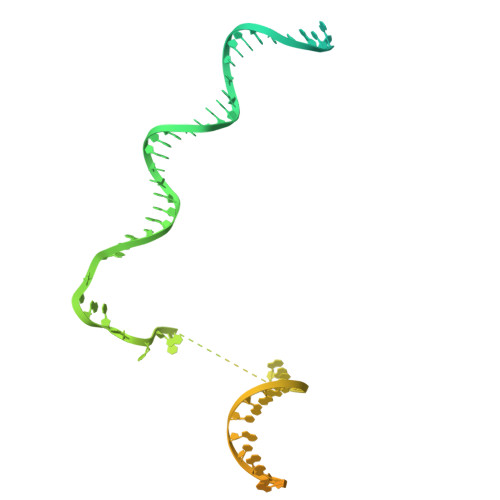
Transcription in bacteria is a multi-step process. In the first step, contacts between RNA polymerase and the promoter DNA must be established for transcription initiation to begin, but then these contacts must be broken for the enzyme to transition into the elongation phase. Single-molecule and biochemical observations report that promoter escape is a highly regulated and sometimes rate-limiting step in the transcription cycle; however, the structural mechanisms of promoter escape remain obscure. Promoter escape also serves as the target for the clinically important antibiotic rifampicin, used to treat tuberculosis. Here, we present seven distinct intermediates showing the structural details of M. tuberculosis RNA polymerase initial transcribing complexes and promoter escape, using a de novo cryo-electron microscopy approach. We describe the structural rearrangements that RNA polymerase undergoes to clear the promoter, including those required to release the initiation factor, σ, providing a structural account for decades of biochemical observations. These structures and supporting biochemistry provide a model of promoter escape, a universal step in the transcription cycle, with conformations that may be used to develop Rifampicin alternatives.
- Laboratory of Molecular Pathogenesis, The Rockefeller University, New York, NY, USA.
Organizational Affiliation: