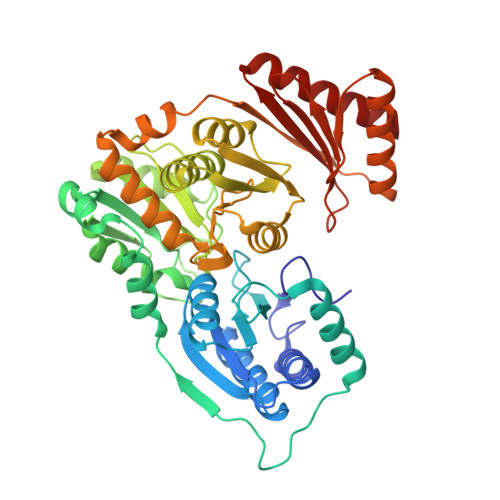
A Bifunctional Phosphoglucomutase/Phosphomannomutase from Thermococcus kodakarensis : Biophysical Analysis and Cryo-EM Structure.
Naz, Z., Rathore, I., Saleem, M., Rahman, M., Wlodawer, A., Rashid, N.(2025) Biomolecules 15
- PubMed: 40149855
- DOI: https://doi.org/10.3390/biom15030319
- Primary Citation of Related Structures:
9DU5 - PubMed Abstract:
Phosphoglucomutase (EC 5.4.2.2., PGM), a key enzyme of glycogenolysis and glycogenesis, catalyzes the interconversion of glucose 1-phosphate and glucose 6-phosphate, whereas phosphomannomutase (EC 5.4.2.8., PMM) transfers the phosphate group from the 1' to the 6', or from the 6' to the 1' position in mannose phosphate. However, in the hyperthermophilic archaeon Thermococcus kodakarensis , a single gene, Tk1108 , encodes a protein with both PGM and PMM activities. Here, we report biophysical analysis and the 2.45 Å resolution cryo-EM structure of this novel enzyme. Our results demonstrate a specific arrangement of the four subunits in the quaternary structure, displaying a distinct catalytic cleft required for the bifunctional activity at extremely high temperatures. To the best of our knowledge, this is the first biophysical characterization and cryo-EM structure elucidation of a thermostable, bifunctional PGM/PMM.
- School of Biological Sciences, University of the Punjab, Lahore 54590, Pakistan.
Organizational Affiliation: