Cryo-EM structure of the USP1-UAF1-Ubiquitin-VS complex
Wylie, A.A.To be published.
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| WD repeat-containing protein 48 | 677 | Homo sapiens | Mutation(s): 0 Gene Names: WDR48, KIAA1449, UAF1 | 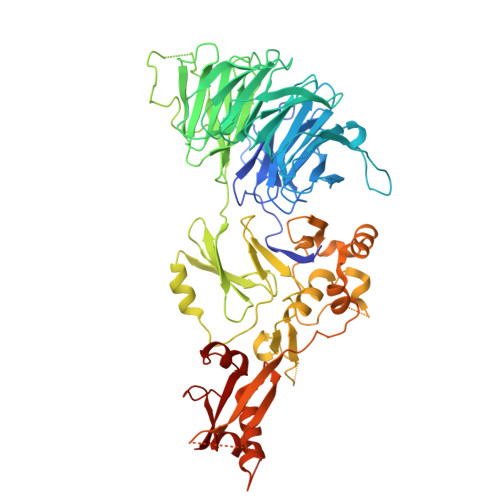 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for Q8TAF3 (Homo sapiens) Explore Q8TAF3 Go to UniProtKB: Q8TAF3 | |||||
PHAROS: Q8TAF3 GTEx: ENSG00000114742 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | Q8TAF3 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin carboxyl-terminal hydrolase 1 | 785 | Homo sapiens | Mutation(s): 0 Gene Names: USP1 EC: 3.4.19.12 | 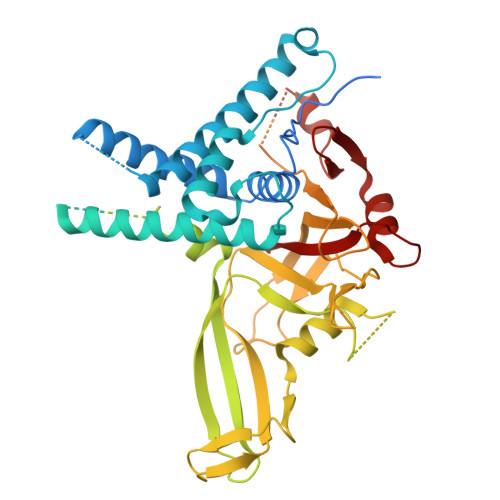 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O94782 (Homo sapiens) Explore O94782 Go to UniProtKB: O94782 | |||||
PHAROS: O94782 GTEx: ENSG00000162607 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O94782 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Entity ID: 3 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Ubiquitin | 75 | Homo sapiens | Mutation(s): 0 Gene Names: UBC | 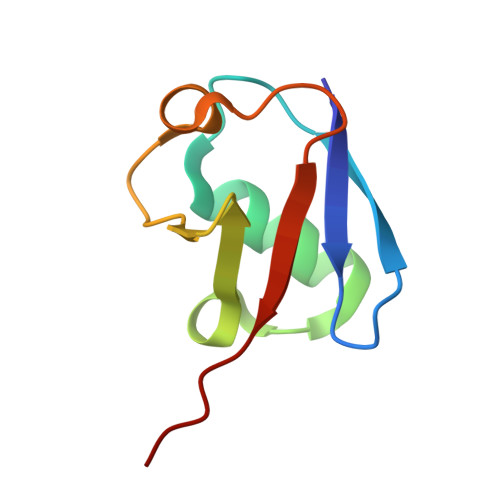 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P0CG48 (Homo sapiens) Explore P0CG48 Go to UniProtKB: P0CG48 | |||||
PHAROS: P0CG48 GTEx: ENSG00000150991 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P0CG48 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 2 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| A1A4Y Query on A1A4Y | E [auth B] | 3-(methanesulfonyl)propan-1-amine C4 H11 N O2 S QUYFSHOLLKVPGX-UHFFFAOYSA-N |  | ||
| ZN Query on ZN | D [auth B] | ZINC ION Zn PTFCDOFLOPIGGS-UHFFFAOYSA-N |  | ||
| Funding Organization | Location | Grant Number |
|---|---|---|
| Not funded | -- |