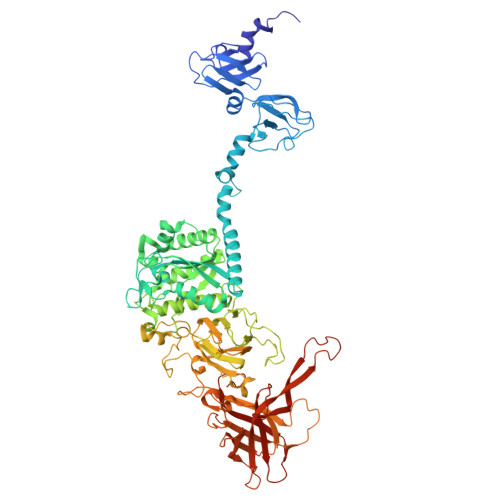
Moo19 and B2: Structures of Schitoviridae podophages with T = 9 geometry and tailspikes with esterase activity.
Subramanian, S., Bergland Drarvik, S.M., Tinney, K.R., Doore, S.M., Parent, K.N.(2024) Sci Adv 10: eadt0022-eadt0022
- PubMed: 39693418
- DOI: https://doi.org/10.1126/sciadv.adt0022
- Primary Citation of Related Structures:
9D7Z, 9D80, 9D81, 9D82, 9D83, 9D84 - PubMed Abstract:
Podophages are, by far, the least well studied of all the bacteriophages. Despite being classified together due to their short, noncontractile tails, there is a huge amount of diversity among members of this group. Of the podophages, the N4-like Schitoviridae family is the least well studied structurally and is quite divergent from well-characterized podophages such as T7 and P22. In this work, we isolate and fully characterize two members of the Schitoviridae family by cryo-electron microscopy, genetics, and biochemistry. We describe the capsid features of Moo19 and B2, including a decoration protein. In addition, we have fully modeled the tail machinery for both phages and identify proteins with esterase activity. Genetic knockouts of the host reveal factors specific for host attachment including key modifications to the O-antigen on the lipopolysaccharide. Moo19 and B2 are both Schitoviridae members, yet some distinct differences in the genome and structure place them into distinct clades.
- Department of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI 48824, USA.
Organizational Affiliation: