NRIP1_133 / RIP140 SxxLxxLL motif coregulator peptide with agonist GW1929 and PPARg LBD
Nemetchek, M.D., Voss, A.H., Hughes, T.S.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Peroxisome proliferator-activated receptor gamma | 276 | Homo sapiens | Mutation(s): 0 Gene Names: PPARG, NR1C3 | 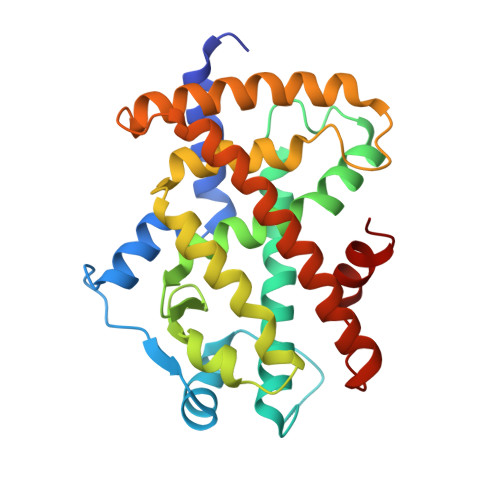 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P37231 (Homo sapiens) Explore P37231 Go to UniProtKB: P37231 | |||||
PHAROS: P37231 GTEx: ENSG00000132170 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P37231 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar proteins by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Nuclear receptor-interacting protein 1 | 23 | Homo sapiens | Mutation(s): 0 Gene Names: NRIP1 | 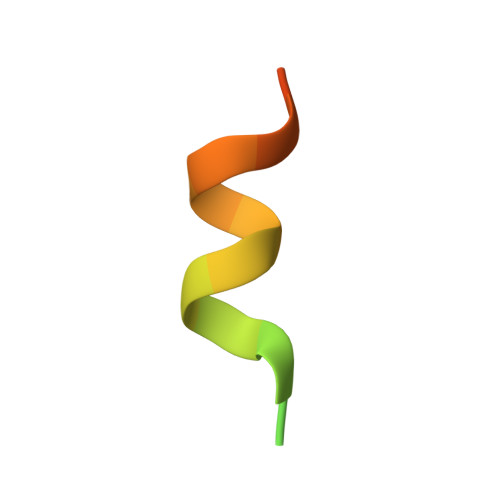 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for P48552 (Homo sapiens) Explore P48552 Go to UniProtKB: P48552 | |||||
PHAROS: P48552 GTEx: ENSG00000180530 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P48552 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| EDK (Subject of Investigation/LOI) Query on EDK | C [auth A] | (2~{S})-3-[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]-2-[[2-(phenylcarbonyl)phenyl]amino]propanoic acid C30 H29 N3 O4 QTQMRBZOBKYXCG-MHZLTWQESA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 57.236 | α = 90 |
| b = 118.815 | β = 90 |
| c = 151.922 | γ = 90 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| PHENIX | phasing |
| XDS | data reduction |
| STARANISO | data scaling |
| autoPROC | data processing |
| Funding Organization | Location | Grant Number |
|---|---|---|
| National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Disease (NIH/NIDDK) | United States | R01DK129646 |
| National Institutes of Health/National Institute of General Medical Sciences (NIH/NIGMS) | United States | P30GM140963 |