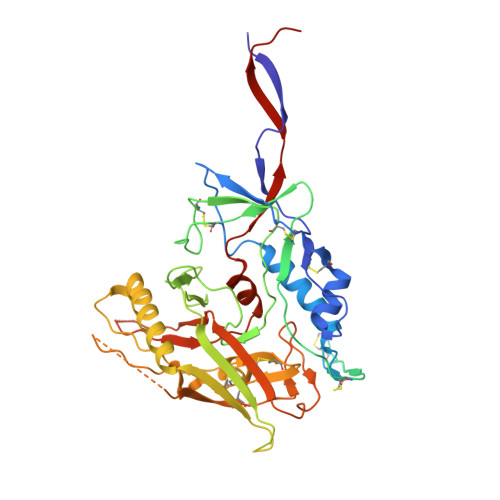
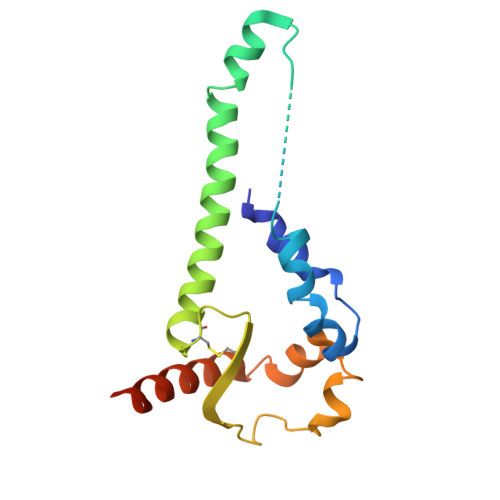
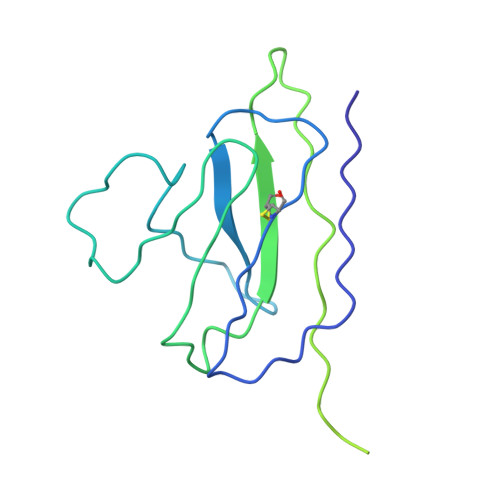
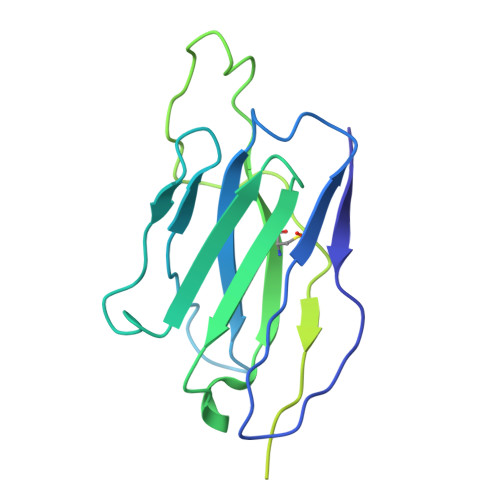
The asymmetric opening of HIV-1 Env by a potent CD4 mimetic enables anti-coreceptor binding site antibodies to mediate ADCC.
Richard, J., Grunst, M.W., Niu, L., Diaz-Salinas, M.A., Tolbert, W.D., Marchitto, L., Zhou, F., Bourassa, C., Yang, D., Chiu, T.J., Chen, H.C., Benlarbi, M., Gottumukkala, S., Li, W., Dionne, K., Belanger, E., Chatterjee, D., Medjahed, H., Hendrickson, W.A., Sodroski, J., Lang, Z.C., Morton, A.J., Huang, R.K., Matthies, D., Smith 3rd, A.B., Mothes, W., Munro, J.B., Pazgier, M., Finzi, A.(2024) bioRxiv
- PubMed: 39253431
- DOI: https://doi.org/10.1101/2024.08.27.609961
- Primary Citation of Related Structures:
9CF5 - PubMed Abstract:
HIV-1 envelope glycoproteins (Env) from primary HIV-1 isolates typically adopt a pretriggered "closed" conformation that resists to CD4-induced (CD4i) non-neutralizing antibodies (nnAbs) mediating antibody-dependent cellular cytotoxicity (ADCC). CD4-mimetic compounds (CD4mcs) "open-up" Env allowing binding of CD4i nnAbs, thereby sensitizing HIV-1-infected cells to ADCC. Two families of CD4i nnAbs, the anti-cluster A and anti-coreceptor binding site (CoRBS) Abs, are required to mediate ADCC in combination with the indane CD4mc BNM-III-170. Recently, new indoline CD4mcs with improved potency and breadth have been described. Here, we show that the lead indoline CD4mc, CJF-III-288, sensitizes HIV-1-infected cells to ADCC mediated by anti-CoRBS Abs alone, contributing to improved ADCC activity. Structural and conformational analyses reveal that CJF-III-288, in combination with anti-CoRBS Abs, potently stabilizes an asymmetric "open" State-3 Env conformation, This Env conformation orients the anti-CoRBS Ab to improve ADCC activity and therapeutic potential.
- Centre de Recherche du CHUM, Montréal, Québec, Canada.
Organizational Affiliation: