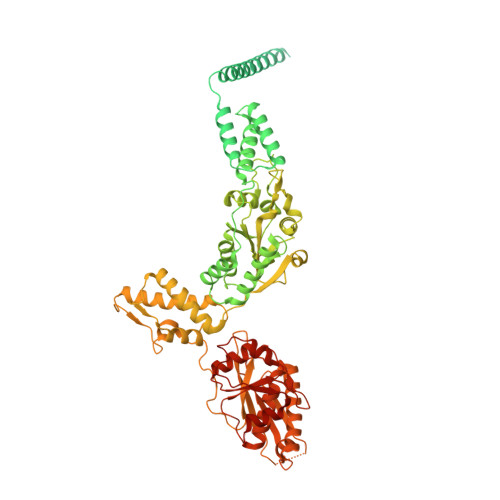
Structural and mechanistic studies on human LONP1 redefine the hand-over-hand translocation mechanism.
Mindrebo, J.T., Lander, G.C.(2024) bioRxiv
- PubMed: 38979310
- DOI: https://doi.org/10.1101/2024.06.24.600538
- Primary Citation of Related Structures:
9CC0, 9CC3 - PubMed Abstract:
AAA+ enzymes use energy from ATP hydrolysis to remodel diverse cellular targets. Structures of substrate-bound AAA+ complexes suggest that these enzymes employ a conserved hand-over-hand mechanism to thread substrates through their central pore. However, the fundamental aspects of the mechanisms governing motor function and substrate processing within specific AAA+ families remain unresolved. We used cryo-electron microscopy to structurally interrogate reaction intermediates from in vitro biochemical assays to inform the underlying regulatory mechanisms of the human mitochondrial AAA+ protease, LONP1. Our results demonstrate that substrate binding allosterically regulates proteolytic activity, and that LONP1 can adopt a configuration conducive to substrate translocation even when the ATPases are bound to ADP. These results challenge the conventional understanding of the hand-over-hand translocation mechanism, giving rise to an alternative model that aligns more closely with biochemical and biophysical data on related enzymes like ClpX, ClpA, the 26S proteasome, and Lon protease.
- Department of Integrative Structural and Computational Biology, Scripps Research; La Jolla, CA, USA.
Organizational Affiliation: