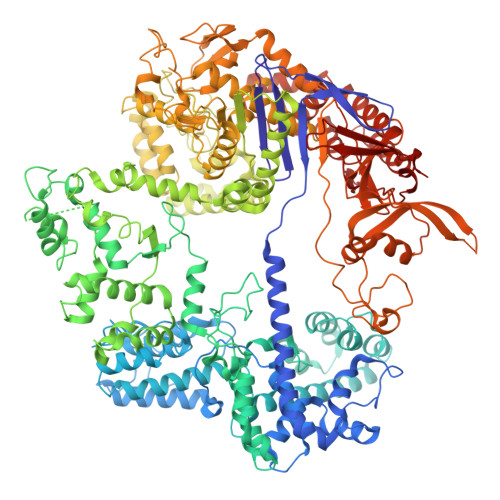
Structural basis of Cas9 DNA interrogation with a 5' truncated sgRNA.
Kiernan, K.A., Kwon, J., Merrill, B.J., Simonovic, M.(2025) Nucleic Acids Res 53
- PubMed: 39657754
- DOI: https://doi.org/10.1093/nar/gkae1164
- Primary Citation of Related Structures:
9C9P, 9CGU - PubMed Abstract:
The efficiency and accuracy of CRISPR-Cas9 targeting varies considerably across genomic targets and remains a persistent issue for using this system in cells. Studies have shown that the use of 5' truncated single guide RNAs (sgRNAs) can reduce the rate of unwanted off-target recognition while still maintaining on-target specificity. However, it is not well-understood how reducing target complementarity enhances specificity or how truncation past 15 nucleotides (nts) prevents full Cas9 activation without compromising on-target binding. Here, we use biochemistry and cryogenic electron microscopy to investigate Cas9 structure and activity when bound to a 14-nt sgRNA. Our structures reveal that the shortened path of the displaced non-target strand (NTS) sterically occludes docking of the HNH L1 linker and prevents proper positioning of the nuclease domains. We show that cleavage inhibition can be alleviated by either artificially melting the protospacer adjacent motif (PAM)-distal duplex or providing a supercoiled substrate. Even though Cas9 forms a stable complex with its target, we find that plasmid cleavage is ∼1000-fold slower with a 14-nt sgRNA than with a full-length 20-nt sgRNA. Our results provide a structural basis for Cas9 target binding with 5' truncated sgRNAs and underline the importance of PAM-distal NTS availability in promoting Cas9 activation.
- Department of Biochemistry and Molecular Genetics, University of Illinois Chicago, 900 S Ashland Ave, Chicago, IL 60607, USA.
Organizational Affiliation: